Difference between revisions of "Fasciolosis"
| (109 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | Also known as: '''''Fascioliasis — Fasciolasis — Fluke | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | + | ==Introduction== |
| − | + | Fasciolosis is a condition of ruminants which causes subclinical and clinical disease leading to ill thrift and deaths. The causative organism is the trematode ''[[Fasciola hepatica]]'' which primarily parasitises the bile ducts of sheep and cattle but may occasionally be found in the horse. ''Lymnaea truncatula'', a mud snail, is the intermediate host of ''[[Fasciola hepatica]]'', and transmission of disease is dependent on the presence of appropriate snail habitats. These habitats are more plentiful in areas of high rainfall, such as the western British Isles. However, infected animals may be found outwith these areas due to the transportation of livestock, or unusual weather patterns. The association of fasciolosis with wetter habitats lends a [[Fasciola hepatica#Epidemiology|seasonal nature to disease outbreaks]], and can help predict the severity of these. | |
| − | |||
| − | |||
| − | |||
| − | + | [[Image:Fasciola hepatica.jpg|thumb|right|150px|''Fasciola hepatica''. Source: Wikimedia Commons; Author:Adam Cuerden(2007)]] | |
| − | ''' | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | In sheep, "acute" disease caused by fluke larvae is the most common presentation, and generally occurs in the wetter Autumn and early Winter months in both lambs and ewes. Fasciolosis in cattle can occur at any time of year and tends to involve adult fluke, causing "chronic" disease. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | '' | + | A slightly larger trematode, ''F. gigantica'', causes a similar condition in tropical regions. |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | ==Signalment== | |
| − | + | Fasciolosis affects both young and adult animals, primarily sheep and cattle. | |
| − | |||
| − | |||
| − | |||
| − | == | + | ==Diagnosis== |
| − | + | A diagnosis can usually be made using clinical findings and the seasonal occurrence of disease, and may be supported by a history of fasciolosis on the farm and post-mortem findings. Certain adjunctive tests may also prove useful. | |
| − | |||
| − | |||
| − | === | + | ===Clinical Signs=== |
| − | + | [[Image:Fasciola hepatica adult.jpg|thumb|right|150px|''Fasciola hepatica'' adults from a horse - Castellà Veterinary Parasitology Universitat Autònoma de Barcelona]] | |
| − | + | ====Sheep==== | |
| − | + | In sheep, fasciolosis may present as acute, chronic, or infrequently sub-acute manifestations. | |
| − | |||
| − | |||
| − | + | '''Acute''' fasciolosis usually occurs between September and December and is caused by large numbers of immature ''[[Fasciola hepatica]]'' migrating through the liver parenchyma and causing massive damage. It arises within around two to six weeks of ingestion of metacercariae. If sheep are not exposed to at-risk pasture until later in the year, acute fasciolosis may occur as late as the following Feburary. Hepatic damage caused by migration of fluke larvae gives clinical signs including lethargy, pallor, dyspnoea and death in both young and adult animals. Handling of sheep may cause liver rupture and sudden death, and sudden death may also occur due to [[Infectious Necrotic Hepatitis|Black's disease]] (''[[Clostridium novyi]]'' type B) or [[Bacillary Haemoglobinuria|bacillary haemoglobinuria]] (''[[Clostridium haemolyticum|Clostridium novyi'' type D]]) in unvaccinated sheep. This is a result of necrosis caused by larval migration within the liver: anaerobic conditions are created, enabling multiplication of clostridial organisms and thus toxin production. | |
| − | + | '''Chronic''' fasciolosis in sheep is caused by adult flukes in the bile ducts and is usually seen in February and March, 4-5 months after ingestion of metacercariae. However, cases may present in early summer if snails become infected during the winter. Progressive weight loss over weeks to months results in poor body condition, and anorexia is often seen. As adult flukes feed on blood and are capable of consuming 0.5ml each per day, [[Regenerative and Non-Regenerative Anaemias|anaemia]] and pallor frequently occur in chronic fasciolosis. Initially, this regenerative anaemia is normochromic, but becomes hypochromic as iron reserves are depleted. Hypoalbuminaemia may also result from whole blood loss, and from reduced hepatic production. This gives a reduced plasma oncotic pressure, leading to ascites and/or submandibular oedema in advanced cases. | |
| − | |||
| + | In some cases, sub-acute fasciolosis may occur if infection has occurred over a prolonged period. In these instances, disease is caused by both adult flukes and larvae and ill thrift, lethargy, dyspnoea is seen from around December to March. | ||
| − | + | In addition to these presentations, ''[[Fasciola hepatica]]'' has subclinical effects on sheep. Fleece weight and fibre quality are affected by even small fluke burdens, and there is some evidence that lambing percentage and lamb growth rates may be negatively influenced. Condemnation of affected livers at slaughter also causes economic losses. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | a) | + | ====Cattle==== |
| + | In cattle, fasciolosis is usually a disease of calves occuring between winter and spring, but may affect any animal at any time of year. Disease is usually chronic (caused by adult flukes) and signs tend to be less severe than in sheep. Poor nutrition and gastrointestinal parasitism do however exacerbate disease. As in sheep, subclinical losses include liver condemnation following slaughter and extended finishing times. Carcass values and milk yield may be reduced in beef and dairy cattle respectively. Milk quality may also be poorer in ''[[Fasciola hepatica]]'' infection. | ||
| − | + | ===Laboratory Tests=== | |
| + | A simple and effective test for ''[[Fasciola hepatica]]'' infection is examination of the faeces for fluke eggs. These are large, ovoid and golden brown, but may be few in number and thus difficult to find. It must also be remembered that eggs are produced only by adult flukes, and so will not be detectable in acute disease. | ||
| − | + | Measurement of serum glutamate dehydrogenase (GLDH) and/or glutamyl transpeptidase (GGT) levels may prove useful. GLDH is an enzyme released by damaged hepatic cells and becomes elevated within the first few weeks of infection. GGT indicates damage to the epithelial cells lining the bile ducts and rises later in disease, although high levels are maintained for longer. Fluke serology is possible by means of a reliable [[ELISA testing|ELISA test]] that identifies anti-fluke antibodies in the serum or in milk. | |
| − | + | ===Diagnostic Imaging=== | |
| + | Although not commonly performed in the diagnosis of fasciolosis, ultrasonography can show hepatic changes that may be suggestive of fluke. The probe should be applied immediately caudal to the costal arch, approximately two-thirds of the way down the right hand side. This positioning may reveal an excess of peritoneal exudate and fibrinous adhesions between the liver and the small intestine or the body wall. The liver is seen to be enlarged, and the capsule appears hyperechoic due to fibrin deposition. The liver parenchyma also exhibits changes, appearing diffusely granular, rather than uniformly hypoechoic. | ||
| − | + | ===Biopsy=== | |
| + | Biopsy or histopathology may reveal necrotic areas of liver, haemorrhage, hyperplastic bile ducts or indeed the presence of ''[[Fasciola hepatica]]'' within the section. | ||
| − | + | ===Pathology=== | |
| + | [[Image:Fasciola hepatica - bile duct.jpg|thumb|right|150px|Bile duct fibrosis due to chronic ''Fasciola hepatica'' infestation. Source: Wikimedia Commons; Author: Flukeman]] | ||
| + | The pathology of fasciolosis is similar in both sheep and cattle, although the species have a different incidence of acute and chronic disease, as described above. | ||
| − | + | In '''acute''' fasciolosis, immature flukes grow and migrate within the liver parenchyma, causing necrotic tracts and haemorrhage. On post-mortem, the liver is enlarged and a fibrinous peritonitis may be be present. Fibrin tags are often particularly apparent on the ventral lobe. Histologically, tracts are seen as areas of degenerate hepatocytes and haemorrhage, which may later become infiltrated with eosinophils and lymphocytes. In the long term, these areas will become fibrosed. In sub-acute fasciolosis, the liver again is enlarged on post-mortem, and haemorrhagic tracts can be seen. | |
| + | '''Chronic''' fasciolosis is associated with damage to the bile ducts by adult flukes. On post-mortem, the liver is distorted by areas of fibrosis caused by the migration of the original immature flukes. The bile ducts are dilated, and flukes may be expressed from within. The gall bladder may also be enlarged. The walls of the bile ducts may be ulcerated and haemorrhagic with areas of epithelial hyperplasia. The walls eventually become fibrosed and may calcify in cattle. Calcified bile ducts can be seen protruding from the liver surface - this is known as "pipe stem liver". | ||
| − | == | + | ==Treatment== |
| − | + | Due to the reliance of disease transmission on appropriate snail habitats and therefore weather, it has been possible to develop models to predict the occurrence of fasciolosis to help its control within flocks and herds. These models evaluate the soil moisture content from May to October by considering rainfall patterns and evapo-transpiration, weighted for season. Although June is a particularly influential month in these models, a drought in late summer can reverse predictions of potentially high snail density, and so forecasts should not be issued prematurely. A complicating factor in the prediction of fasciolosis is the fact that snail density is insufficient for disease in the absence of infection (i.e. deposited fluke eggs), and so forecasts generated must be interpreted in the context of local biology. | |
| − | |||
| − | |||
| − | |||
| + | Anthelmintic drugs are used in the control of fasciolosis. Not all flukicides are effective against each parasitic developmental stage, and so some may not be suitable for use in an outbreak of acute disease. Triclabendazole, a benzimidazole, is the flukicide with the broadest spectrum of activity against both immature and adult ''[[Fasciola hepatica]]'' and is therefore used to control acute disease. However, triclabendazole-resistant fluke populations are beginning to emerge. Albendazole, closantel, clorsulon and nitroxanyl all have a narrower spectrum of activity, primarily against adult fluke. | ||
| − | + | There are two objectives to anthelmintic control of fluke in sheep and cattle. The first is to limit shedding of fluke eggs onto snail habitats, which is achieved by the use of any adulticidal drug in late winter/early spring. The second aim is to protect animals grazing metacercariae-contaminated pasture against fluke infection, and the approach to this is not so simplistic. Here, the choice of drug, the timing of treatment and dosing interval is dependent on: a) whether acute or chronic disease is the (anticipated) problem; b) the likely intensity of challenge, based on local knowledge or fluke forecasting and c) the persistence of the selected drug. When treating for chronic fasciolosis it is important to provide good quality nutrition following anthelmintic dosing, and response and need for further treatment can be monitored using faecal egg counts. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | Previously, molluscicides have been employed to control fasciolosis and were successful. However, these are now infrequently used. This is in part due to the need for application before the likely impact of fluke can be predicted, potentially creating an unnecessary cost. Molluscides can also be difficult to use effectively since careful application is needed to avoid rapid recolonisation of treated land from any habitat that has been missed. | |
| − | |||
| − | |||
| − | + | The nature of fluke transmission means that is it possible to use environmental strategies to help control. Recognition of potential snail habitats allows areas to be fenced off or drained to limit snail numbers. Alternatively, animals may simply be grazed away from wet or boggy areas during high-risk periods. | |
| − | |||
| + | Finally, a [[Vaccines|vaccine]] against fluke in cattle is currently in development. This is a recombinant vaccine which is thought to provide around 70% protection by stimulating a range of immune responses not normally seen in chronically infected cattle, for example the [[T cell differentiation#TH1 Cells|Th1 response]]. | ||
| − | === | + | ==Prognosis== |
| − | |||
| − | + | With effective treatment, the prognosis for fasciolosis is reasonable, but this is dependent upon the severity of disease at diagnosis, for example the prognosis for chronic infections is better than that for acute fasciolosis. Clearly strategies aimed at prevention provide the optimum prognosis for herd/flock health. | |
| − | + | ==Links== | |
| − | + | *''[[Fasciola hepatica]]''. | |
| + | *[http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/22702.htm The Merck Veterinary Manual - ''Fasciola hepatica''] | ||
| + | *[http://www.who.int/neglected_diseases/diseases/fascioliasis/en/index.html World Health Organisation - Fascioliasis] | ||
| + | *[http://www.sac.ac.uk/mainrep/pdfs/tn557liverfluke.pdf Treatment and Control of Liver Fluke (Scottish Agricultural College)] | ||
| − | + | ==Test yourself with the Trematodes Flashcards== | |
| − | + | [[Trematodes_Flashcards|Trematodes Flashcards]] | |
| − | |||
| − | + | ==Literature Search== | |
| + | [[File:CABI logo.jpg|left|90px]] | ||
| − | === | + | Use these links to find recent scientific publications via CAB Abstracts (log in required unless accessing from a subscribing organisation). |
| − | + | <br><br><br> | |
| + | [http://www.cabdirect.org/search.html?rowId=1&options1=AND&q1=%28title%3A%28%22Fasciolosis%22%29+OR+title%3A%28Fascioliasis%29+OR+title%3A%28Fasciolasis+%29+OR+title%3A%28%22liver+fluke%22%29+OR+title%3A%28%22fasciola+hepatica%22%29%29+AND+od%3A%28cattle%29&occuring1=freetext&rowId=2&options2=AND&q2=&occuring2=freetext&rowId=3&options3=AND&q3=&occuring3=freetext&publishedstart=2000&publishedend=yyyy&calendarInput=yyyy-mm-dd&la=any&it=any&show=all&x=56&y=10 Fasciolosis in cattle publications since 2000] | ||
| − | + | [http://www.cabdirect.org/search.html?rowId=1&options1=AND&q1=%28%28title%3A%28%22Fasciolosis%22%29+OR+title%3A%28Fascioliasis%29+OR+title%3A%28Fasciolasis+%29+OR+title%3A%28%22liver+fluke%22%29+OR+title%3A%28%22fasciola+hepatica%22%29%29+AND+od%3A%28sheep%29%29&occuring1=freetext&rowId=2&options2=AND&q2=&occuring2=freetext&rowId=3&options3=AND&q3=&occuring3=freetext&publishedstart=2000&publishedend=yyyy&calendarInput=yyyy-mm-dd&la=any&it=any&show=all&x=68&y=9 Fasciolosis in sheep publiations since 2000] | |
| − | + | ==References== | |
| − | + | #Merck & Co (2008) '''The Merck Veterinary Manual (Eighth Edition)''', ''Merial''. | |
| + | #Aitken, I D (2007) '''Diseases of Sheep''', ''Blackwell''. | ||
| + | #Pugh, D G (2002) '''Sheep and Goat Medicine''', ''Elsevier Health Sciences''. | ||
| + | #Taylor, M A et al (2007) '''Veterinary Parasitology (Third Edition)''', ''Wiley-Blackwell''. | ||
| − | |||
| − | + | {{review}} | |
| − | + | [[Category:Liver Diseases - Cattle]] [[Category:Liver Diseases - Sheep]] [[Category:Brian Aldridge reviewing]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 14:24, 6 May 2011
Also known as: Fascioliasis — Fasciolasis — Fluke
Introduction
Fasciolosis is a condition of ruminants which causes subclinical and clinical disease leading to ill thrift and deaths. The causative organism is the trematode Fasciola hepatica which primarily parasitises the bile ducts of sheep and cattle but may occasionally be found in the horse. Lymnaea truncatula, a mud snail, is the intermediate host of Fasciola hepatica, and transmission of disease is dependent on the presence of appropriate snail habitats. These habitats are more plentiful in areas of high rainfall, such as the western British Isles. However, infected animals may be found outwith these areas due to the transportation of livestock, or unusual weather patterns. The association of fasciolosis with wetter habitats lends a seasonal nature to disease outbreaks, and can help predict the severity of these.
In sheep, "acute" disease caused by fluke larvae is the most common presentation, and generally occurs in the wetter Autumn and early Winter months in both lambs and ewes. Fasciolosis in cattle can occur at any time of year and tends to involve adult fluke, causing "chronic" disease.
A slightly larger trematode, F. gigantica, causes a similar condition in tropical regions.
Signalment
Fasciolosis affects both young and adult animals, primarily sheep and cattle.
Diagnosis
A diagnosis can usually be made using clinical findings and the seasonal occurrence of disease, and may be supported by a history of fasciolosis on the farm and post-mortem findings. Certain adjunctive tests may also prove useful.
Clinical Signs
Sheep
In sheep, fasciolosis may present as acute, chronic, or infrequently sub-acute manifestations.
Acute fasciolosis usually occurs between September and December and is caused by large numbers of immature Fasciola hepatica migrating through the liver parenchyma and causing massive damage. It arises within around two to six weeks of ingestion of metacercariae. If sheep are not exposed to at-risk pasture until later in the year, acute fasciolosis may occur as late as the following Feburary. Hepatic damage caused by migration of fluke larvae gives clinical signs including lethargy, pallor, dyspnoea and death in both young and adult animals. Handling of sheep may cause liver rupture and sudden death, and sudden death may also occur due to Black's disease (Clostridium novyi type B) or bacillary haemoglobinuria (Clostridium novyi type D) in unvaccinated sheep. This is a result of necrosis caused by larval migration within the liver: anaerobic conditions are created, enabling multiplication of clostridial organisms and thus toxin production.
Chronic fasciolosis in sheep is caused by adult flukes in the bile ducts and is usually seen in February and March, 4-5 months after ingestion of metacercariae. However, cases may present in early summer if snails become infected during the winter. Progressive weight loss over weeks to months results in poor body condition, and anorexia is often seen. As adult flukes feed on blood and are capable of consuming 0.5ml each per day, anaemia and pallor frequently occur in chronic fasciolosis. Initially, this regenerative anaemia is normochromic, but becomes hypochromic as iron reserves are depleted. Hypoalbuminaemia may also result from whole blood loss, and from reduced hepatic production. This gives a reduced plasma oncotic pressure, leading to ascites and/or submandibular oedema in advanced cases.
In some cases, sub-acute fasciolosis may occur if infection has occurred over a prolonged period. In these instances, disease is caused by both adult flukes and larvae and ill thrift, lethargy, dyspnoea is seen from around December to March.
In addition to these presentations, Fasciola hepatica has subclinical effects on sheep. Fleece weight and fibre quality are affected by even small fluke burdens, and there is some evidence that lambing percentage and lamb growth rates may be negatively influenced. Condemnation of affected livers at slaughter also causes economic losses.
Cattle
In cattle, fasciolosis is usually a disease of calves occuring between winter and spring, but may affect any animal at any time of year. Disease is usually chronic (caused by adult flukes) and signs tend to be less severe than in sheep. Poor nutrition and gastrointestinal parasitism do however exacerbate disease. As in sheep, subclinical losses include liver condemnation following slaughter and extended finishing times. Carcass values and milk yield may be reduced in beef and dairy cattle respectively. Milk quality may also be poorer in Fasciola hepatica infection.
Laboratory Tests
A simple and effective test for Fasciola hepatica infection is examination of the faeces for fluke eggs. These are large, ovoid and golden brown, but may be few in number and thus difficult to find. It must also be remembered that eggs are produced only by adult flukes, and so will not be detectable in acute disease.
Measurement of serum glutamate dehydrogenase (GLDH) and/or glutamyl transpeptidase (GGT) levels may prove useful. GLDH is an enzyme released by damaged hepatic cells and becomes elevated within the first few weeks of infection. GGT indicates damage to the epithelial cells lining the bile ducts and rises later in disease, although high levels are maintained for longer. Fluke serology is possible by means of a reliable ELISA test that identifies anti-fluke antibodies in the serum or in milk.
Diagnostic Imaging
Although not commonly performed in the diagnosis of fasciolosis, ultrasonography can show hepatic changes that may be suggestive of fluke. The probe should be applied immediately caudal to the costal arch, approximately two-thirds of the way down the right hand side. This positioning may reveal an excess of peritoneal exudate and fibrinous adhesions between the liver and the small intestine or the body wall. The liver is seen to be enlarged, and the capsule appears hyperechoic due to fibrin deposition. The liver parenchyma also exhibits changes, appearing diffusely granular, rather than uniformly hypoechoic.
Biopsy
Biopsy or histopathology may reveal necrotic areas of liver, haemorrhage, hyperplastic bile ducts or indeed the presence of Fasciola hepatica within the section.
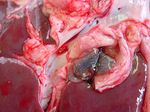
Pathology
The pathology of fasciolosis is similar in both sheep and cattle, although the species have a different incidence of acute and chronic disease, as described above.
In acute fasciolosis, immature flukes grow and migrate within the liver parenchyma, causing necrotic tracts and haemorrhage. On post-mortem, the liver is enlarged and a fibrinous peritonitis may be be present. Fibrin tags are often particularly apparent on the ventral lobe. Histologically, tracts are seen as areas of degenerate hepatocytes and haemorrhage, which may later become infiltrated with eosinophils and lymphocytes. In the long term, these areas will become fibrosed. In sub-acute fasciolosis, the liver again is enlarged on post-mortem, and haemorrhagic tracts can be seen.
Chronic fasciolosis is associated with damage to the bile ducts by adult flukes. On post-mortem, the liver is distorted by areas of fibrosis caused by the migration of the original immature flukes. The bile ducts are dilated, and flukes may be expressed from within. The gall bladder may also be enlarged. The walls of the bile ducts may be ulcerated and haemorrhagic with areas of epithelial hyperplasia. The walls eventually become fibrosed and may calcify in cattle. Calcified bile ducts can be seen protruding from the liver surface - this is known as "pipe stem liver".
Treatment
Due to the reliance of disease transmission on appropriate snail habitats and therefore weather, it has been possible to develop models to predict the occurrence of fasciolosis to help its control within flocks and herds. These models evaluate the soil moisture content from May to October by considering rainfall patterns and evapo-transpiration, weighted for season. Although June is a particularly influential month in these models, a drought in late summer can reverse predictions of potentially high snail density, and so forecasts should not be issued prematurely. A complicating factor in the prediction of fasciolosis is the fact that snail density is insufficient for disease in the absence of infection (i.e. deposited fluke eggs), and so forecasts generated must be interpreted in the context of local biology.
Anthelmintic drugs are used in the control of fasciolosis. Not all flukicides are effective against each parasitic developmental stage, and so some may not be suitable for use in an outbreak of acute disease. Triclabendazole, a benzimidazole, is the flukicide with the broadest spectrum of activity against both immature and adult Fasciola hepatica and is therefore used to control acute disease. However, triclabendazole-resistant fluke populations are beginning to emerge. Albendazole, closantel, clorsulon and nitroxanyl all have a narrower spectrum of activity, primarily against adult fluke.
There are two objectives to anthelmintic control of fluke in sheep and cattle. The first is to limit shedding of fluke eggs onto snail habitats, which is achieved by the use of any adulticidal drug in late winter/early spring. The second aim is to protect animals grazing metacercariae-contaminated pasture against fluke infection, and the approach to this is not so simplistic. Here, the choice of drug, the timing of treatment and dosing interval is dependent on: a) whether acute or chronic disease is the (anticipated) problem; b) the likely intensity of challenge, based on local knowledge or fluke forecasting and c) the persistence of the selected drug. When treating for chronic fasciolosis it is important to provide good quality nutrition following anthelmintic dosing, and response and need for further treatment can be monitored using faecal egg counts.
Previously, molluscicides have been employed to control fasciolosis and were successful. However, these are now infrequently used. This is in part due to the need for application before the likely impact of fluke can be predicted, potentially creating an unnecessary cost. Molluscides can also be difficult to use effectively since careful application is needed to avoid rapid recolonisation of treated land from any habitat that has been missed.
The nature of fluke transmission means that is it possible to use environmental strategies to help control. Recognition of potential snail habitats allows areas to be fenced off or drained to limit snail numbers. Alternatively, animals may simply be grazed away from wet or boggy areas during high-risk periods.
Finally, a vaccine against fluke in cattle is currently in development. This is a recombinant vaccine which is thought to provide around 70% protection by stimulating a range of immune responses not normally seen in chronically infected cattle, for example the Th1 response.
Prognosis
With effective treatment, the prognosis for fasciolosis is reasonable, but this is dependent upon the severity of disease at diagnosis, for example the prognosis for chronic infections is better than that for acute fasciolosis. Clearly strategies aimed at prevention provide the optimum prognosis for herd/flock health.
Links
Test yourself with the Trematodes Flashcards
Literature Search
Use these links to find recent scientific publications via CAB Abstracts (log in required unless accessing from a subscribing organisation).
Fasciolosis in cattle publications since 2000
References
- Merck & Co (2008) The Merck Veterinary Manual (Eighth Edition), Merial.
- Aitken, I D (2007) Diseases of Sheep, Blackwell.
- Pugh, D G (2002) Sheep and Goat Medicine, Elsevier Health Sciences.
- Taylor, M A et al (2007) Veterinary Parasitology (Third Edition), Wiley-Blackwell.
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |