T cell differentiation
Introduction
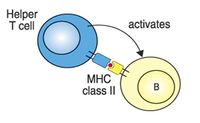
T cells are long lived and are involved in cell mediated immunity. Functionally they are divided by the expression of CD4+ or CD8+ markers. CD4+ T helper cells recognise antigens bound to MHC II complexes and are involved with the control of intracellular and extracellular pathogens; they can interact with CD8+, NK and dendritic cells or with B cells. Cytotoxic CD8+ T cells recognise the MHC I complex and destroy infected or neoplastic cells.
Within the blood and lymphoid organs the majority of T cells are antigen-naive T cells; only a small proportion are memory T cells. Naive T cells have yet to encounter antigen and can only be activated by antigen that is presented by dendritic cells. After initial antigenic activation, naïve T-cells develop into an intermediate stage cell called the TH0 cell which can then be activated by any antigen-presenting cell, e.g. Dendritic cells, macrophages or B cells.
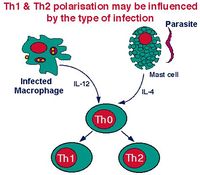
The TH0 cells have the capacity to differentiate into TH1, TH2 cells and a very recently described subtype TH17 cells. The type of cell that develops depends on the antigen presenting cell type. Macrophages cause the TH0 cell to develop into a TH1 cell induced by IL-12 production following macrophage-antigen interaction. B cells cause the TH0 cell to develop into a TH2 cell induced by IL-10 production following B cell-antigen interaction. On antigenic stimulation the TH1 or TH2 cells become activated, undergo clonal expansion and secrete a range of different cytokines. The third most recently described subset, TH17, form in the presence of IL-6 and TGF-β which are produced in the prescence of infection, and by either of the Antigen Presenting Cells (APCs). The importance of CD4+ TH cells is very clear in immunity. An example of a disease that targets CD4+ T cells is the Human Immunodeficieny Viruses (HIV) and Simian Immunodeficieny Viruses (SIV) which, when the CD4+ T cells are overwhelmed, causes Advanced Immunodeficiency Syndrome (AIDS).
For any one cell the cytokine-secreting activation state is short-lived, lasting between 4 - 40 hours. After this time these cells either die or mature into the long-lived memory cells. The proliferation of T cells continues until the presentation of antigen ceases.
Dendritic Cells
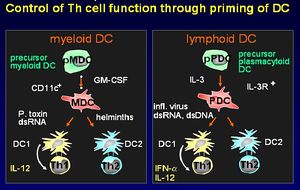
There are two different lineages of dendritic cells:
- From myeloid precursor cells
- From plasmacytoid precursor cells
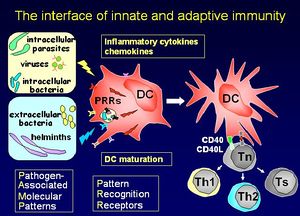
Dendritic cells stimulate a primary T cell response; they migrate through tissues, track to T cell dependent areas of the lymph nodes and cluster with the T cells. Dendritic cells have unique capabilities to take up antigen by pathways involving phagocytosis, macropinocytosis and clathrin-coated pits. The cell-surface antigen phenotype distinguishes the dendritic cell from Monocytes/macrophages and B cells. Their main function is priming T helper cells. They produce cell signaling cytokine molecules known as chemokines.
Maturation signals
- Exogenous
- Bacteria or their products (LPS, LTA, lipoproteins)
- Viruses or their products (dsRNA, G-RSV)
- Protozoa or their products
- Helminths (SEA, ES 62)
- Endogenous
- Inflammatory mediators (IL-1/TNFα, hsp, FcR)
- Immune cells (CD40L, CD47, FasL)
Antigen Presentation
Circulating monocytes differentiate to form immature dendritic cells called Langerhans Cells
Langerhans cells sample the tissue fluid by endocytosis:
- Foreign organisms are internalised
- Within the dendritic cells, antigen is digested to peptides
- Some of the peptides formed bind to the cell’s MHC molecules
The Langerhans cells leave the epithelium and travel via the afferent lymph flow. They are now known as Veiled Cells. Veiled cells enter the paracortical region of the lymph node where they present antigen to the T cells. They are now known as Interdigitating Dendritic Cells.
TH1 Cells
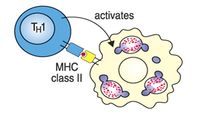
TH1 cells help macrophages digest bacteria - the organisms are contained in cellular vesicles.
TH1 cells secrete a range of cytokines, including:
- IL-2, which induces proliferation of both CD4+ and CD8+ T-cells. This stimulation of T cell proliferation is the main function of the TH1 cell.
- Interferon gamma (IFNγ) which activates tissue macrophages and is the principal effector mechanism in the defence against intracellular bacteria and parasites such as Mycobacteria, Brucella, Rickettsia Leishmania, Coccidia, and Babesia. IFNγ activates macrophages and stimulates them to produce enzymes triggering intracellular killing mechanisms - specifically:
- Superoxide dismutase and myeloperoxidase that produce H2O2 and trigger the "superoxide burst".
- Nitric oxide synthase which produces nitric oxide.
This is another example of the immune system working through the innate immune response, and this can even act to suppress antibody synthesis.
TH2 Cells
TH2 cells help B cells produce antibody where the organism is present in tissue fluid. The TH2 population influences B cell activation, proliferation and immunoglobulin production. TH2 T cells also secrete a range of cytokines:
- IL-4 which stimulates B cell growth and induces the heavy chain switch from IgM to IgG , IgA and IgE, as well as proliferation of basophils and mast cells. IL-4 can inhibit some T cell responses.
- IL-5 which activates B cells and stimulates a high rate of proliferation. IL-5 also promotes immunoglobulin synthesis and the proliferation and differentiation of eosinophils.
- IL-6 also activates B cells, stimulates a high rate of proliferation and promotes immunoglobulin synthesis.
Common Functions of TH1 and TH2 Cells
Both TH1 and TH2 cells produce IL-3 and granulocyte-macrophage colony stimulating factor (GM-CSF). These act to activate and induce proliferation of neutrophils and macrophages. Neutrophils are the major phagocytic cells in the blood and the principal cells in acute inflammatory lesions whose function is chiefly the body's defence against extracellular bacteria. One of the major biological functions therefore of the activation of either TH subset is cytokine-controlled reactive haematopoiesis.
TH17 Cells
Still under investigation
The TH17 cells form when TH0 cells are challenged with IL-6 and TGF-β to produce a number of cytokines that enhance the innate immune response [1]. The cytokines produced enhance the extravasation and chemotaxis of neutrophils to the site of infection, in the aim of combating extracellular bacteria. These cytokines include:
- IL-17
- IL-17F
- IL-6
- TNFα
- IL-21
- IL-22
- IL-23
Importantly they do not produce:
- IFNγ normally associated with TH1 cells
- IL-4 normally associated with TH2 cells
Cytotoxic T-Cells
Cytotoxic T cells kill virus infected cells where the organisms are contained in the cell cytoplasm. Viruses are obligate intracellular pathogens that use the host cell machinery for pathogen protein synthesis; viral peptides associate with MHC class I and are expressed on the cell surface. CD8+ cytotoxic T lymphocytes (CTL) recognise the antigen-MHC complex. Cytotoxic T-cells secrete a pattern of cytokines similar to that of TH1 cells:
- IFNγ but not IL-2. The IFNγ shifts the balance of the immune response in favour of TH1 cells and there is therefore an increased level of T-cell proliferation. The initiation of the immune response via CTL leads to the selective proliferation of CTL which enhances the main mechanism of killing virally-infected cells.
Killing Mechanism
The CTl killing mechanism is initiated by direct CTL-target cell contact.
- The cells involved bind by antigen/MHC class I-TcR interaction. This allows the CTL's intracellular granules to be localised at the area of contact - the granules contain most of the molecules responsible for cytotoxicity.
- Direct cell contact stimulates the release of the granule contents into the area of contact between the two cells. The granules contain two groups of cytotoxic molecules.
- Perforin, which is structurally related to the complement component, C9 and forms pores in the cell membrane.
- Granzymes, which are proteolytic enzymes that target cell nucleases and cause programmed cell death.
T-Cell Activation
T cells function only after recent activation by an antigen.
- CD4 binds MHC class II - CD4+ T-cells recognise antigen only in association with MHC class II.
- CD8 binds MHC class I - CD8+ T-cells recognise antigen only in association with MHC class I.
Activation of T cells requires two distinct signals:
- Signal 1 is the interaction of the TcR with the antigenic peptide/MHC complex on the antigen presenting cell.
- Signal 2 is the interaction of CD28 on the T cells with its ligand, CD80, on the antigen-presenting cell (APC). APC expression of CD80 only occurs after the engagement of pattern recognition on Fc receptors or activation by the cytokines Interferon, IL-1β or TNFα.
Signal 2 only occurs after the recognition of DANGER.
Activation Scenarios
1. No signal 1:
- T cell is not activated as there is no antigen.
2. Both signal 1 and signal 2
- T cell is activated into clonal expansion and produces cytokines or becomes cytotoxic.
3. Signal 1 but no signal 2
- T-cell is triggered into apoptosis and dies.
- This is the basis of "clonal deletion" and is a major mechanism of the development of tolerance. It ensures that T-cells do not react with self (non-dangerous) antigens.
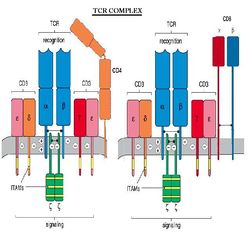
TCR Complex
The T cell Receptor, or TCR is always associated with CD3, forming what is referred to as the TCR complex. TCR is expressed on the surface of T cells in a noncovalent association with a complex of transmembrane polypeptides.
CD3 contains 3 distinct polypeptide chains that are expressed exclusively on T cells: γ, ε, and δ. These molecules are members of the Ig superfamily - the ε chain associates with both γ and δ - and they play a 'chaperone' role in transporting newly synthesized TCR molecules to the cell surface. CD3 also contains 2 identical chains: ζ and 16 kDa, which are found on T cells, macrophages and NK cells. Mice also can have an ε (eta) form.
Response to Activation
The response of the T cells to obtaining Signals 1 and 2 is to express the receptor for the cytokine interleukin-2 (IL-2) and CD4+ T-cells secrete IL-2. The final trigger for clonal expansion is the engagement of IL-2R with IL-2 from any activated CD4+ T-cell. IL-2 produced by a CD4+ cell may also stimulate clonal expansion of the CD4+ cell.
T-Helper Cell Function
The function of T helper cells is to regulate the immune response. The cytokines they secrete exert their influence on other cell populations; most of the different effector cells of the immune system are affected by one or more of the cytokines secreted by TH cells.
TH cells secrete cytokines for only a short period after they have been activated; the range of cytokines that TH cells secrete after activation chiefly determines their function. Different T-helper cell subpopulations (TH1, TH2 and TH17 cells) secrete different sets of cytokines.
References
- ↑ Korn, T., Bettelli, E., Oukka, M. and Kuchroo, V.K. (2009) IL-17 and Th17 Cells. Annual Reviews of Immunology 27: pp.485-517.
| Originally funded by the RVC Jim Bee Award 2007 |
Error in widget FBRecommend: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a697fe1784f5_55520532 Error in widget google+: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a697fe1e2854_14740624 Error in widget TwitterTweet: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a697fe2413f4_07664451
|
| WikiVet® Introduction - Help WikiVet - Report a Problem |