Difference between revisions of "Artificial Insemination - Anatomy & Physiology"
Jump to navigation
Jump to search
m (Text replace - "[[Reproductive_Behaviour_-_Copulatory_Behaviour_- Anatomy & Physiology" to "[[Copulatory Behaviour - Anatomy & Physiology") |
|||
| Line 1: | Line 1: | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[Image:Artificial Insemination pig.jpg|thumb|right|150px|<p>Artificial Insemination of the Sow</p><sup>Courtesy of Proff.Watson ©RVC 2008</sup>]] | [[Image:Artificial Insemination pig.jpg|thumb|right|150px|<p>Artificial Insemination of the Sow</p><sup>Courtesy of Proff.Watson ©RVC 2008</sup>]] | ||
| Line 196: | Line 187: | ||
| − | [[Category:Reproductive | + | [[Category:Reproductive Technologies]] |
Revision as of 17:48, 1 December 2010
Introduction
Artificial insemination involves:
- Collection of semen from the male
- Preservation and extension of sperm
- Insemination of the female
A large proportion of the UK dairy and beef herds are bred by artificial insemination. It is also common practice in turkeys, pigs and horses. Success rates are very dependent on good insemination technique, which can be carried out by an inseminator service or DIY. Generally, success rates are the same or better than natural service.
Semen Evaluation
- Determine ejaculate volume.
- Estimate the percentage displaying progressive motility (linear motion) at 37◦C.
- Determine sperm concentration.
- Total sperm in ejaculate = ejaculate volume x sperm (ml)
- This determines how many insemination doses are possible from each ejaculate.
- >60% motile sperm = good quality
- <50% motile sperm = discard, especially if it is intended to be frozen
Seminal Extenders
- Extend the number of sperm in the original ejaculate.
- Extend the life of sperm.
- Must be isotonic
- Must minimize cold damage.
- The cell membrane of spermatozoon is sensitive to sudden drops in temperature 'cold shock'.
- Slow, controlled cooling of sperm is important because it minimizes stress on the cell membrane by lowering temperatures gradually.
- A low storage temperature reduces metabolism by about 50% for each 10◦C decline.
- Enables conservation of their fixed amount of metabolic energy.
- Must be a good buffer
- Typical buffers include Sodium Citrate and Sodium Phosphate.
- When the goal is to extend the semen for a sustained period of time (1 week-years) a cryoprotectant is required.
- Protects cells against the cold damage that would occur at 0-50◦C.
- Classified as cell-penetrating (glycerol, DMSO) and non-penetrating (milk protein, egg yolk lipoproteins).
- Glycerol is the dominant cryoprotectant for frozen sperm.
- Milk protein and egg yolk lipoproteins will also provide nutrients.
- Sperm have no anabolic capacity, so cannot synthesize materials for energy and repair.
- Vitality is totally dependent on the environment in which they are suspended.
- Nutrients need to be supplied to maintain metabolism.
- Major nutrients are fructose and glucose.
- Sperm can convert glucose to fructose and metabolize it to fuel motility.
- Semen contains microorganisms which can grow in the ideal environment provided by the extender.
- Antibiotics are normally added to prevent microbial growth.
- Include penicillin, liquamycin, linco-spectin and streptomycin.
Semen Preservation
Short Term
- For short term use:
- Fresh liquid semen is used after seminal extension.
- In most species this can be cooled and stored at 5◦C for several days-1 week.
- In swine 17-18◦C is optimal.
Long Term
- Widespread distribution and long-term use:
- Frozen semen
- Freezing and thawing compromises sperm viability in all species.
- Fertility drops much faster either side of ovulation with frozen semen, especially in the sow.
- Hence, artificial insemination with frozen semen is rare in the sow.
Advantages
- Enables widespread use of good sires and the spread of valuable genetic material to even small farms.
- Permits crossbreeding more economically.
- Eliminates dangerous males.
- Research tool for reproductive pathology.
- Allows introduction of new genetics to be seen quicly in a population.
- Enables the use of semen (frozen) even after the donor is dead, thus preserving selected lines.
- Allows the use of semen from incapacitated males.
- Reduces the risk of spreading disease.
- Leads to improved herd performance.
- Reduced risk of injury to handlers.
- Dramatically improved genetic quality and diversity in many animals.
- Semen can be stored for optimal timing of insemination.
- Transporting semen is much cheaper than it would be to transport animals.
Disadvantages
- If one animal can sire many offspring, it will make many animals redundant.
- Foals conceived by AI are not permitted in any stud book and are not allowed to race.
- Legislation is needed to provide necessary safeguards.
- High level of knowledge and skill are required.
- Need for government intervention and support.
- Slight risk of inseminating with the wrong semen.
- Animals which need to be inseminated very soon after ovulation (horses) require veterinary input. This may be expensive.
- Frozen semen cannot be used successfully in all species and fertility rates are lower with frozen semen than when fresh semen is used.
- Optimum fertility allows only a small window of time and transport of fresh semen is difficult.
Insemination Methods
Cattle
- Many doses from a single ejaculate.
- Inseminate 6 hours post-oestrus.
- Success rate is 70% from a single insemination (same as natural service)
- Farmers inseminate the herd themselves.
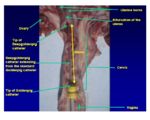
- A hand is used to grasp the cervix of the cow and a delivery pipette is inserted through the cervix into the uterine body which is felt with the index finger.
- Allows further advance of the semen than natural insemination would reach (anterior vagina).
- Only 2-8 million live sperm are required in the semen to achieve fertility in cattle.
Sheep
- The ewe has a convex cervix with overlapping folds. Insemination is into the internal os of the cervix.
- 0.25 ml or less is delivered by pipette.
- 70-75% conception rate is achieved with fresh semen, but only 5-15% with frozen semen due to the site of delivery being so far from the site of fertilization.
Laparoscopic insemination
- Small numbers of sperm (20 million) are injected into the uterine horns, compared with the 200 million in cervical insemination.
- Achieves good fertility,especially for frozen semen.
- Incise a small hole in the abdominal wall.
- Place the ewe head down, feet up so that the uterine tissue is visible.
- Inject through the uterine wall directly into the uterine horn.
- But, this is expensive and can only be carried out by a vet in the UK under local anaesthesia.
Swine
- Minimum volume 25-50ml
- Normally 80ml is delivered through the cervix to reach the uterus by gravitational flow.
- Uses 2-3 billion sperm per insemination (60 billion in a normal ejaculate), so is relatively inefficient only giving ~20 inseminations per ejaculate.
Horses
- Gloved, lubricated hand is inserted directly into the vagina.
- Index finger is used to guide the insemination pipette into the cervical lumen.
- Marker used to guage the depth of insemination.