Hypoadrenocorticism
| This article is still under construction. |
| Also known as: | Addison's disease |
Description
Addison's disease occurs due to a failure to produce adequate amounts of glucocorticoid and mineralocorticoid hormones from the adrenal cortex. The majority of cases are primary and occur due to an autoimmune response directed at the endocrine cells of the adrenal glands, resulting in adrenocortical atrophy. This is an example of a type IV (delayed type) hypersensitivity response. Smaller numbers of primary cases are caused by haemorrhage, necrosis or neoplasia within the adrenal glands. Secondary hypoadrenocorticism occurs due to a reduction in the secretion of ACTH from the anterior pituitary gland.
Primary Hypoadrenocorticism
There are several possible causes of primary hypoadrenocorticism, including:
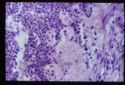
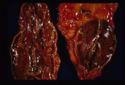
- Adrenal atrophy is thought to be an autoimmune disease caused by a type IV immune response. Affected animals have an increased risk of developing other immune-mediated diseases, including immune-mediated haemolytic anaemia. Grossly, the adrenal glands are small, difficult to locate and they are dark brown on cut sections. Histological analysis reveals the presence of an infiltrate of lymphocytes, plasma cells and macrophages.
- Adrenal necrosis may be caused by certain infections or it may be a sequel to other metabolic diseases. In horses, salmonellosis may cause adrenal necrosis in horses but, in small animals, necrosis is more likely to occur due to myoarteritis in uraemic animals resulting in ischaemia of the adrenal gland. Idiopathic necrosis may occur with no apparent cause. Grossly, the glands contain areas of red haemorrhage with yellow necrotic foci.
- Bilateral adrenalectomy or mitotane therapy may cause iatrogenic hypoadrenocorticism. Mitotane, which is used in the treatment of hyperadrenocorticism (Cushing's disease) selectively destroys the zona fasciculata and reticularis while sparing the essential zona glomerulosa. Iatrogenic Addison's disease is a common sequel of its use.
Secondary Hypoadrenocortisism
Deficient pituitary secretion of ACTH. Often iatrogenic due to withdrawal of glucocorticoid treatment. Prolonged high dose treatment induce adrenal atrophy due to the effect of negative feedback on the pituitary. The withdrawal of drug must be gradual to allow to adrenal gland to return to function over a period of time. Usually little effect on mineralocorticoids as ACTH has little trophic effect on their production.
Pathophysiology
The glucococorticoid hormone cortisol enables animals to cope with stress while the minerlaocorticoid aldosterone plays a critical role in the regulation of sodium and potassium concentrations and of extracellular fluid volume. Aldosterone normally acts to increase sodium reabsorption and potassium excretion in the kidney so deficient aldosterone secretion will result in hyponatraemia, hypochloraemia and hyperkalaemia. Since they are unable to regulate their body sodium concentration, Addisonian animals become severely dehydrated and hypovolaemic, reducing the perfusion of peripheral tissues. During an Addisonian crisis, this can result in gastro-intestinal haemorrhage and allow translocation of bacteria across the gut barrier.
Deficiency of cortisol results in hypoglycaemia (as cortisol usually antagonises the action of insulin), increased circulating levels of lymphocytes and eosinophils and increased skin pigmentation. This latter syndrome occurs as low levels of glucocorticoids allow increased ACTH production as negative feedback on the pituitary is removed or decreased. As ACTH is released, so is MSH (Melanocyte Stimulating Hormone), increasing the pigmentation of the skin in chronic cases of hypoadrenocorticism.
Diagnosis
Clinical Signs
Addison's disease may present acutely as an Addisonian crisis or it may cause chronic disease over several months before it is diagnosed. Acutely, affected animals present in hypovolaemic shock with severe dehydration and bradycardia (caused by hyperkalaemia). This feature of the crisis is extremely suggestive of Addison's disease as hypovolaemic animals would usually be expected to be tachycardic. As with any cause of hypovolaemia, the peripheral pulse is often weak, the capillary refill time is prolonged and the heart sounds may not be clearly audible on auscultation.
Addisonian animals may suffer vomiting as part of an acute crisis or as a chronic intermittent syndrome. In the chronic form of the disease, animals are frequently anorexic and weak.
Laboratory Tests
Complete analysis of blood samples is indicated for animals presenting acutely and for those that have a history of chronic intermittent vomiting and anorexia. The following changes may be documented:
- Haemoconcentration in an acute crisis due to rapid dehydration.
- Non-regenerative anaemia in chronic form as glucocorticoid deficiency decreases erythropoeisis. Since it is possible for animals to undergo an acute crisis after suffering from hypoadrenocorticism for several weeks, any anaemia may be masked by the haemoconcentration that occurs due to hypovolaemia.
- Electrolyte imbalances occur in 90% of patients, typically hyperkalaemia, hyponatraemia and hypochloraemia. The sodium: potassium ratio of a normal animal is >27: 1 but this falls to <25: 1 in Addisonian animals.
- Pre-renal azotaemia is common as hypovolaemic animals are unable to maintain adequate renal perfusion.
- Hypoglycaemia is a common finding in animals presenting in Addisonian crisis.
- Eosinophilia and lymphocytosis occur due to the deficiency in glucocorticoids. In such a severely ill animal, a stress leucogram would usually be expected so this pattern is highly suggestive of hypoadrenocorticism. An elevated blood urea concentration may also occur due to gastro-intestinal haemorrhage which is a common finding in animals with Addison's disease.
Other Tests
Additional tests are available to confirm the diagnosis and assess the need for further supportive treatment. An ACTH stimulation test should be performed as soon as possible in animals presenting acutely. The pre-stimulation sample can be taken immediately, the ACTH (tetracosactin) is administered and the post- sample is collected after 1.5-2 hours. Although the results of the test may not be available for 24-48 hours, it is very useful to confirm the diagnosis when acute renal failure is considered to be a major differential diagnosis. A positive result is recorded if the level of cortisol is initially low and if this fails to respond to the injection of ACTH.
Since hyperkalaemia has significant effects on the membrane potential of excitable tissues, it is useful to perform an electrocardiogram (ECG) to assess the function of the heart. Findings consistent with hyperkalaemia include small or absent P waves (indicating sino-atrial standstill), wide and small QRS complexes, short Q-T intervals, peaked T waves and long P-R intervals.
Diagnostic Imaging
Plain radiographs of the chest and abdomen may show signs of hypovolaemia, including microcardia, pulmonary underperfusion and microhepatica. Animals with Addison's disease may also develop megaoesophagus due to muscle weakness. Ultrasound scans of the adrenal glands may be indicated to detect any evident pathological process that may account for the hypoadrenocorticism.
Treatment
Stabilisation
Since collpased, bradycardic animals are unlikely to survive for long, urgent intervention is required to stabilise these patients. Intra-venous 0.9% sodium chloride (saline) solution should be provided at shock rates to restore normovolaemia and begin to correct the electrolyte imbalances. Additional glucose can be added to fluids in hypoglycaemic animals but blood glucose levels should be monitored closely if this is undertaken.
When the diagnosis has been made with some certainty, intra-venous glucocorticoid replacement therapy can be initiated together with a mineralocorticoid. Dexamethasone is the corticosteroid of choice as it has greater mineralocorticoid activity than other products. Although there is an intra-muscular injectable mineralocorticoid (desoxycorticosterone acetate) available in the USA, this is not usually required for stabilisation.
Gastro-protectant drugs (such as sucralfate, ranitidine or omeprazole) may be administered to vomiting animals and antibiotics should be provided to any animals that develop pyrexia.
Management
In the long term, animals with Addison's disease require only a mineralocorticoid on a daily basis with intermittent courses of glucocorticoids during times of stress. The mineralocorticoid fludrocortisone acetate is available in an oral preparation and this is used most commonly for management.
During times of stress, courses of corticosteroids (e.g. prednisolone) should be provided and table salt should be added to the diet. Water should be avaiable at all time and blood electrolyte levels should be monitored at regular intervals to ensure that the correct dose of fludrocortisone is used.
Prognosis
The prognosis for long term survival is excellent for animals with hypoadrenocorticism provided that any crisis is controlled.