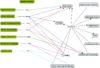
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
|
|
In general
- Some viruses are thought to induce modifications of the pulmonary defences by:
- Damaging the upper respiratory tract, thereby facilitating bacterial attachment and colonisation, with reduced mucociliary clearance
- Decreasing surfactant levels by destroying Type 2 pneuMonocytes - WikiBlood
- Impairing the phagocytic ability of alveolar macrophages
In Dogs
Canine distemper
- Caused by a morbillivirus
- Rhinitis
- Although many organs can be affected by CDV, a relatively constant feature is the respiratory signs which occur in varying severity
- A syndrome of catharral oculonasal discharge, pharyngitis and bronchitis is relatively common in the initial stages
- Since one of the primary sites of action of this virus is lymphoid tissue, the resultant immunosuppression -> predisposition to secondary bacterial infection
- May cause interstitial pneumonia where inclusions are found within alveolar macrophages
- Gross pathology:
- Oedematous lungs, diffuse interstitial pneumonia
- Micro pathology:
- Necrosis of pneumocytes, necrotising bronchiolitis, alveolar oedema, thickening of alveolar walls and type II pneumocyte hyperplasia
Parainfluenza- 2
- Caused by an parainfluenza- 2 virus
- Rhinitis, tracheobronchitis
Infectious canine tracheitis
- Synonyms: Kennel cough, Infectious tracheobronchitis
- tracheitis, bronchitis
- Multiple agents implicated:
- Symptoms are of a persistent, non-productive cough
- Persistent tracheobronchial inflammation
- The outcomes is generally recovery (may persist >3 weeks), but extension to chronic bronchitis or cranioventral bronchopneumonia may occur
- In severe cases can extend to serous/mucopurulent rhinitis
- Lesions are neither specific nor always significant (catarrhal / mucopurulent tracheobronchitis)
- Enlarged tonsils and retropharyngeal lymph nodes
Canine adenovirus
- Adenoviridae
- Usually mild bronchointerstitial pneumonia, necrosis of bronchiolar and alveolar epithelium, oedema, type II pneumocyte hyperplasia
- May cause necrotising bronchiolitis in immune-deficient dogs (distemper)
- Can be associated with kennel cough described above
Canine herpes virus
- Caused by canine herpes virus 1
- Part of fading puppy syndrome
- Presents with necrotising rhinotracheitis and secondary bronchopneumonia in older dogs
- Seems to be common subclinically
- (CRCV)
- Shown to be involved in an outbreak of disease in large kennels with rapidly changing population and high incidence of respiratory disease
- Erles, K., Toomey, C. et al.(2003) "Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease." Virology 310(2):216-223
In Cats
Feline viral rhinotracheitis
- Caused by a herpesvirus
- Tends to be more rhinitis than tracheitis, may extend to sinusitis
- Feline herpesvirus -1
- One of the causes of Feline viral rhinotracheitis
- Viruses and bacteria are involved in the complex. The most frequent aetiologic agent is FHV-1, and less frequently feline calicivirus and/or Chlamydophila psittaci (NB: previously called Chlamydia psittaci var felis)
- One of the causes of Feline viral rhinotracheitis
- All three agents infect URT respiratory epithelium, although FHV-1 has the highest affinity for this epithelium
- Feline calicivirus more frequently infects the oral mucosa -> ulcerative stomatitis
- C.psittaci more frequently infects the conjunctival epithelium -> chronic conjunctivitis
- Infection of the respiratory epithelium by FHV-1 results in a typical neutrophilic rhinitis with intraepitheial intranuclear eosinophilic inclusion bodies
- Uncomplicated cases resolve in 2-3 weeks
- FHV-1 remains latent in the trigeminal ganglion, and can reactivate at times of stress
- Can infect the cornea -> ulcerative keratitis
- Mortality may be high in young kittens, debilitated and immune-suppressed animals, usually associated with secondary bacterial infection.
Feline calicivirus
- Causative agent: feline calicivirus
- Rhinitis,
- Suggested in the presence of ulceration of the dorsal and lateral edges of the tongue, hard palate and external nares
- Lesions present may include interstitial pneumonia with necrotising bronchiolitis
- Also see Feline viral rhinotracheitis above
In Horses
Equine rhinovirus
- Causative agent: equine rhinovirus
- Rhinitis
- Most common in young horses
- May cause acute upper respiratory disease
Equine influenza
- Causative agent: equine influenza virus (Orthomyxovirus)
- Rhinitis,
- Pathogenesis:
- Inhalation -> replication in epithelial cells of upper and lower airways (attaches via haemaglutinin spike and gains entry into cell) -> neuramidase alters efficiency of mucociliary apparatus
- Sloughing of the affected area
- Severity of signs will depend on dose of virus
- Acute tracheobronchitis with coughing, and fever
- May be accompanied by secondary bacterial infections
- No viraemia
- In severe cases may cause bronchointerstitial pneumonia
- Secondary invaders are usually Streptococci
Equine rhinopneumonitis
- Causative agent: equine herpesvirus type 1 and type 4
- Primary viral lesions in nasal mucosa and lungs
- Mild, transient bronchointerstitial pneumonia
- Latent infection acting as a reservoir
- Sites of latency: bronchial lymph nodes and trigeminal ganglia
- Replicates in upper respiratory tract epithelium
- Disseminated to lower respiratory tract
- Transported to other organs in T-lymphocytes - viraemia up to 3 weeks
- Vasculitis, abortion
- May be accompanied by secondary bacterial infection
Equine viral arteritis (EVA)
- Causative agent: equine arterivirus
- Rhinitis, peripheral oedema, bronchitis/bronchiolitis, conjunctivitis, periorbital oedema
- Replicates in macrophages and endothelial cells
- Disseminates via the circulatory system causing necrotising arteritis
- Interstitial pneumonia
- Transmitted by respiratory and venereal routes through direct contact with infected horse or its secretions
- Stallion are a reservoir of infection as they are chronic shedders
Equine adenovirus
- Adenoviridae
- May cause necrotising bronchiolitis in immune-deficient foals (Arabian foals)
- Grossly:
- Atelectasis and consolidation of lobules in cranioventral region
- Mucopurulent exudate in airways
- Histologically:
- Severe bronchiolitis, necrotising -> proliferative
- Bronchiolar obstruction by sloughed debri and neutrophils -> alveolar atelectasis
- May lead to secondary bacterial infections
African horse sickness
- Caused by orbivirus, family reoviridae
- Respiratory distress or cardiovascular failure
- Rapid death due to massive pulmonary oedema
- Hydrothorax may also develop
- Large amounts of froth present in airways
Hendra virus
- Causative agent equine morbillivirus
- Oedematous lungs with distention of pleura
- Micro: diffuse alveolar oedema, syncytial cells
In Cattle
Infectious bovine rhinotracheitis (IBR)
- Causative agent is bovine herpesvirus, type 1
- URT infection with serous nasal discharge, increases respiratory rate, coughing and moderate fever,from nasal mucosa down to bronchioles
- Rhinitis, pharyngitis, laryngitis, tracheitis, bronchiolitis
- Morbidity is high, mortality is low
- Highly infectious URT disease of cattle
- Spread by movement of animals, aerosol transmission - requires close contact between animals
- Early stages (only first few days) may show intracellular inclusions in the respiratory mucosal epithelial cells
- Leading to neutrophilic inflammation of varying severity.... serous -> catarrhal -> purulent
- With secondary bacterial infection (eg: Pasturella spp., Mycoplasma spp., Fusobacterium necrophorum) can lead to fibrinous to necrotizing inflammation; mucosal sloughing, ulceration... pyrexia, dyspnoea ... inhalation pneumonia... death
- Underlying hyperaemic inflammatory response
- Can become latent following primary infection
- Clinical signs:nasal discharge, sneezing, coughing, lacrimation, and increased respiratory rate
- Clinical disease most severe in young calves - can develop mucosal ulcerative lesions in the oesophagus and forestomachs and viraemia with multiorgan infection
- Cause of abortion >5 months of gestation
- May contribute to Enzootic pneumonia of calves
Parainfluenza- 3
- Causative agent: parainfluenza- 3 virus (PI3)
- On its own causes rhinitis
- Often part of multi-aetiology disease complex (e.g. Enzootic pneumonia of calves), often followed by Pasteurella sp. obscuring viral origin
- Replicates in airway epithelial cells and results in an initial bronchitis -> bronchiolitis -> extension into alveoli, causing bronchointerstitial pneumonia
- Early stages may show intracytoplasmic inclusions
- The resulting exudate is predominantly neutrophilic
- Positive confirmation lies in a Fluorescent Antibody Test (FAT) to the specific virus on frozen sections of tissue
Bovine adenovirus
- Causative agent: bovine adenovirus
- Rhinitis
- May contribute to Enzootic pneumonia of calves
Respiratory syncytial virus
- Causative agent Respiratory syncytial virus (RSV), synonym: bovine RSV (BRSV)
- Outbreaks of RSV associated disease usually occur associated with winter housing
- Gross pathology in severe cases
- Cranioventral atelectasis and consolidation
- Interstitial emphysema
- More prominent in the caudal lung lobes
- Results from bronchoconstriction which results in airway obstruction - this constriction is thought to arise from mast cell degranulation and histamine release
- Histologically
- Acute bronchiolitis, characteristic of the bronchiolar response is the formation of syncytial giant cells (formed by proliferating bronchiolar epithelial cells which may contain intracytoplasmic inclusion bodies), alveolar epithelium sometimes affected
- Obstruction of bronchioles by exudate - these may later become obliterated by the fibrous tissue of organisation
- May contribute to Enzootic pneumonia of calves
Bovine rhinovirus
- Causative agent: bovine rhinovirus
- May cause mild respiratory disease
In Sheep
Maedi Visna
- Caused by a retrovirus
- The respiratory from of the disease caused by maedi-visna virus (Maedi) is also called lymphoid interstitial pneumonia
- Transmitted by close contact and via milk
- The pulmonary lesions develop very slowly hence this disease is uncommon in sheep < 2 years old
- Increased respiratory rate upon exertion, loss of weight
- Remains in Monocytes - WikiBlood and macrophages
- Gross findings
- Severe interstitial pneumonia
- Lungs fail to collapse properly on opening the chest and can weigh more than twice the normal weight
- Impressions of the ribs remain on the visceral pleura
- Lungs are a mottled grey/ tan colour - the lesions can vary from irregular grey speckling to homogeneous grey consolidation
- Rubbery in consistence
- Diaphragmatic lobes most affected
- Associated bronchial and mediastinal lymph nodes are often enlarged
- Histologically
- Major features are extensive lymphoid proliferation around perivascular, peribronchial and peribronchiolar sheaths associated with pulmonary lymphatics
- Many of these areas contain germinal centres and smooth muscle hyperplasia (in walls of terminal bronchioles and alveoli)
Parainfluenza -3
- As in cattle
Pulmonary adenomatosis
- See neoplasia
In Goats
Caprine Arthritis-Encephalitis (CAE)
- Caused by retrovirus (lentivirus) similar to Maedi Visna in sheep described above
- Two forms:
- Non-suppurative leukoencephalomyelitis in young goats and kids
- Chronic, non-suppurative arthritis-synovitis in adult goats
- Also causes interstitial pneumonia which tends to be obscured by other clinical signs
- Gross pathology:
- Mainly caudal lobes
- Lungs are firm, grey-pink with grey-white focal lesions on cut surface
- Micro pathology:
- Thickened alveolar wall
- Lymphocyte infiltration and type II pneumocyte hyperplasia
- Can be confused with or coexisting with Parasitic pneumonia
In Pigs
Inclusion body rhinitis
- Herpesviridae, porcine cytomegalovirus
- Disease of suckling piglets 1-5 wks of age
- Clinical signs: those associated with acute/subacute rhinitis (ie: serous nasal discharge, progressing to catarrhal or purulent discharge with time and secondary bacterial infections; sneezing; pyrexia), fever in young piglets (3-8wks old)
- May progress to sinusitis, otitis media or pneumonia
- Morbitity high, mortality low
- Gross pathology - catarrhal discharge becoming purulent (secondary infection)
- Histology:
- Large basophilic intranuclear inclusion bodies in the surface and subepithelium of nasal and sinus glandular epithelium with lymphocytic infiltration of the mucosa
- Bursting of nucleus with cell necrosis and sloughing of necrotic epithelium
- Can develop viraemic stage, with inclusions and focal necrotising lesions in other organs eg: renal tubular epithelium
- Usually younger piglets, can die during this phase
- Usually resolves if uncomplicated but rhinitis may persist if secondary infection is present
- May persist in pulmonary macrophages
Swine influenza
- Caused by Swine influenza virus
- Rhinitis, may progress to pneumonia
- Clinical signs: pyrexia, lethargy, skin erythema, anorexia, severe cough and sneezing, dyspnoea, conjunctivitis, pregnant sows may abort
- Grossly:
- Tracheobronchitis, airway obstruction -> atelectasis
- Pleura normal or covered with serous or serofibrinous exudate
- Pleural cavity filled with excess fluid
- Lung lesions
- Clear demarcation of lesions in cranial and middle lobes
- Interstitial pneumonia
- Histologically:
- Acute inflammation of mucosa of trachea and bronchi
- Zoonotic
- Circumstantial evidence of mutation from human strain
- Migrating ascarids thought to precipitate the disease, reservoir of infection in earthworms containing infected lungworm larvae
Porcine reproductive and respiratory syndrome
- The syndrome is caused by a small enveloped RNA virus which belongs to the new Arteriviridae group
- Replicates in and destroys macrophages and endothelial cells causing vasculitis -> viraemia -> virus shedding (nasal secretions, faeces)
- Clinical signs: respiratory and reproductive failure, weaned pigs, tachypnoea, eyelid oedema, conjunctivitis
- Moderate to severe interstitial pneumonia in the cranial lobe
- Superimposed bacterial infections are common
- Infectious disease in swine that emerged 10 years ago
- Today, PRRS is endemic in many if not all the pig-producing countries
Postweaning multisystemic wasting syndrome (PMWS)
- Caused by a porcine circovirus alone or in combination with porcine parvovirus
- May cause mild interstitial pneumonia, failure of lungs to collpse on opening the thoracic cavity
- Microscopically: thickening of alveolar wall due to type 2 pneumocyte hyperplasia
- Caused by a coronavirus
- Usually mild pneumonia unless complicated by other agents
- Virus replicates in epithelial lining of airways