Equine Protozoal Myeloencephalitis
Also known as: EPM — Equine protozoal myelitis — Equine protozoal encephalomyelitis
Introduction
A progressive, infectious,[1]neurological disease of horses, endemic in the USA[2] and only encountered elsewhere in equids that have travelled in the Americas.[3] Equine protozoal myeloencephalitis (EPM) is one of the most frequently diagnosed neurological conditions in the Western Hemisphere[4] and the principal differential for multifocal, asymmetric progressive central nervous system (CNS) disease.[1] The disease is not contagious.[1]
Aetiology
EPM results from infection of the CNS by the apicomplexan parasite Sarcocystis neurona or, less frequently, its close relative Neospora hughesi.[5][6] These protozoans develop within neurons[4] causing immediate or inflammatory-mediated neuronal damage. The organisms migrate randomly through the brain and spinal cord causing asymmetrical lesions of grey and white matter and thus multifocal lower and upper motor neuron deficits.[1]
Epidemiology
In endemic areas of the United States, around a quarter of referrals for equine neurological disease are attributed to EPM.[7] According to the United States Department of Agriculture, the average incidence is 14 cases per 10,000 horses per year. However, the challenges of obtaining a definitive diagnosis may mean this figure is an underestimate.[4] EPM has been identified in parts of Central and South America, southern Canada and across most of the USA.[4] The disease is noted occasionally in other countries, in horses that have been imported from endemic regions.[8][9] It is likely that these animals carried a silent but persistent infection during transportation. There have been reports of EPM in horses that have not travelled to or from endemic regions,[4] although cross-reacting antigens in immunodiagnostic tests may explain this discrepancy. [4]
The route of infection remains unconfirmed,[1] but there is an increased risk associated with a young age (1-4 years)[10]and autumn months.[11] The reported age range for EPM cases is currently 2 months[2] to 24 years.[12] Thoroughbreds, Standardbreds and Quarterhorses are most frequently affected across the US and Canada.[13] This may relate to a breed predispostion or alternatively, managemental factors associated with these breeds.[14] Showing and racing have been linked to a greater risk of clinical disease.[15] Increasing age and environmental temperature have been associated with an increased seroprevalence of S. neurona.[16] Seroprevalence for this species is typically higher than for N. hughesi.[4]Other risk factors for EPM include the presence of opossums, rats, mice and woodland, increased population density of humans and horses, bedding horses on shavings or wood chips and the use of purchased grain.[4]Case clustering may operate where all the risk factors occur, but the majority of cases appear in isolation.[4]
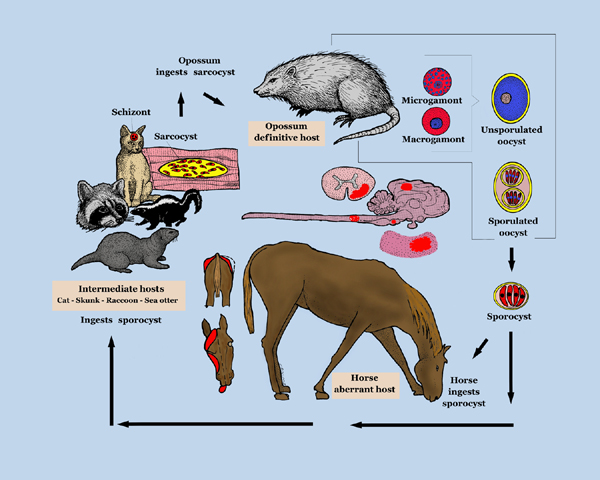
Life Cycle
Infective sporocysts are passed in the faeces of the definitive host and must be ingested by the horse for infection to occur. See the Sarcocystis page for further details of the life cycle of S.neurona.
Pathogenesis
Immune clearance of S.neurona must be, in the large part, very effective, since less than 1% of horses exposed to the protozoan suffer from EPM.[11] Both humoral and cell-mediated immune mechanisms are likely to be significant in the host defence against S.neurona. Antibodies are produced soon after infection and offer some degree of protection.[4] CD8 positive T-cells and their production of IFN-γ are likely to be pivotal in the removal of intracellular stages of the parasite.[4]Factors which promote disease development include parasite dose[17]and, most probably virulence of the protozoal strain. Stress induced by pregnancy, travel, training and showing[18] may have an immunosuppressive effect that encourages infection. Indeed, it has been shown that stress affects the severity of clinical signs seen in natural infections.[19]
The 'Trojan horse' hypothesis suggests that S.neurona meroziotes traverse the blood brain barrier encrypted within leucocytes that have phagocytosed the parasite in the periphery. Once inside the CNS, eggression and infection of other cells results in encephalitis.[20] Other theories include haematogenous spread or direct passage of parasites via the cytoplasm of endothelial cells into the CNS. However, despite extensive histological lesions, few organisms are typically visible in the neural tissues of affected horses. This implies that cytokines may have a considerable role in producing pathological changes.[21] Although the protozoan may induce some degree of immunosuppression in the host[22][23], it is likely that the immune-privilege of the CNS prevents parasite clearance from this site.[4]. The methods by which S.neurona and N.hughesi cause EPM is still debated.
Signalment
Mostly Standardbreds and Thoroughbreds aged 1-6years.[1] Foal infection may be possible.[2]
Clinical Signs
The disease onset may be acute, peracute or chronic. An insidious onset ataxia is most typical and with such cases, the clinical examination may reveal a bright, alert horse, perhaps with some focal muscle atrophy.[4] In all cases, the clinical signs are referable to diffuse focal and multifocal lesions of the white and grey matter of the spinal cord and brain.[3] The three characteristic 'As' (ataxia, asymmetry, atrophy) suggest multifocal or diffuse disease, but are not pathognomonic for EPM.[4]
| Lesion Location | Clinical signs |
| Spinal cord |
|
| Peripheral nerves |
|
| Brainstem (cranial nerve signs) |
|
| Cerebrum, basal nuclei, cerebellum |
|
Lesions of the brainstem, cerebrum or cerebellum are less frequently recognized than those of the spinal cord. Horses with severe EPM may be unable to stand or swallow and, if left untreated, progress to recumbency within 14 days to 6 months.[1] This deterioration may occur smoothly or spasmodically,[26] but is likely to result in death. It has been suggested that rapidly progressive presentations reflect brainstem lesions.[4]
Diagnosis
It is difficult to obtain a definitive antemortem diagnosis of EPM. Certain criteria must be met before such a diagnosis is assigned[4]:
- The relevant clinical signs must be attributable to one or more lesions of the CNS[27]
- Immunodiagnostic tests must confirm exposure to the parasite
- Other differentials with similar presentations should be ruled out wherever possible
- The horse should be resident in or have travelled within the Americas[3]
The primary step in the diagnostic procedure should be to carry out thorough clinical and neurological examinations. [27]
Immunodiagnostic tests
All of these tests aim to confirm exposure to the pathogens of EPM by detecting the presence of antibodies to these parasites.[4] None of these tests is considered a gold standard and they are only supportive. Currently, a definitive diagnosis can only be obtained at postmortem.[27]
- Immunoblot analysis (Western blot) of serum and CSF: senstivity around 90%, specificity 48-89%.[28] Cultured merozoites are used to detect antibodies versus S.neurona-specific proteins. The blood brain barrier does not prevent the passage of antibodies, thus the CSF concentration of a specific antibody will be directly related to its serum concentration.[29] This permeability is likely responsible for many of the weakly false-positive CSF immunoblot tests. Blood contamination during CSF collection or bleeding within the CNS due to trauma or infection might also cause false positives. The CSF titre will be greatly increased during CNS infection as there will be local production of the antibody. One of the difficulties in interpreting immunoblot results is that many horses develop antibodies against S.neurona in the absence of neurological disease.[28] For this reason, testing CSF may be preferable to serum despite the impact that minor blood contamination may have on CSF results.[27] False negative results may arise if horses fail to respond to the specific proteins recognised by the immunoblot. Such cases are rare, so a negative immunoblot result tends to exclude the diagnosis of EPM.[26] Cases that originally test negative should be re-tesed 14-21 days later. In most instances, owing to a substantial incubation period, detectable levels of IgG are present prior to the emergence of clinical signs.[4]
- Whole organism indirect fluorescent antibody test (IFAT): sensitivity around 90%, specificity 97-100%.[28] Serum titres of more than 1:100 and CSF titres of more than 1:5 indicate an active infection. The IFAT is considered to have slightly improved diagnostic efficiency than the immunoblot test[30] but is unable to distinguish between S.neurona and other related nonpathogenic organsims such as S.fayeri.[31] This can lead to false positive results. Compared with the immunblot test, CSF blood contamination has an insignificant effect on the IFAT.[32]An IFAT for N.hughesi is also available from the Universty of California.[4]
- ELISA for antibodies to the snSAG-1 protein: based on an immunodominant surface antigen of S.neurona (SAG-1).[27] Serum titres more than 1:100 suggest an active infection. False negatives are possible as not all S.neurona isolates produce the specific protein.[33] SAG-5 is an alternative surface antigen of S.neurona strains, which is mutually exclusive to SAG-1.[34] Therefore, the ELISA may only be of use where strains of S.neurona expressing SAG-1 predominate.[27]
Other tests
- CSF analysis: to rule out other conditions as stated below. Most horses with EPM have normal CSF. Rarely, an increased total protein or white blood cell count is seen in severe cases.[4] PCR can be used to detect S.neurona DNA in CSF.[2]
- Diclazuril: a positive response to treatment with diclazuril would firmly support a diagnosis of EPM, since the drug has no antimicrobial activity.[35]
- Blood gene expression biomarkers: may be sensitive and specific indicators of early and active disease[36]
Differential Diagnoses
S.neurona can migrate to any region of the CNS[2], thus the differential list comprises almost all diseases of this system.
| Differential | Differentiating signs | Tests to rule out |
| Cervical vertebral malformation (CVM, cervical compressive myelopathy, cervical vertebral instability, cervical stenotic myelopathy, cervical spondylomyelopathy, Wobbler's syndrome). | Symmetrical gait deficits, worse in pelvic limbs[37] with spasticity and dysmetria, good retention of strength, no muscle wasting.[4] NB: can be concurrent with EPM.[38] | Plain lateral radiography of C1 to T1[38], myelography. [39] |
| West Nile encephalitis | Systemically ill, pyrexia. Difficult to differentiate if horse is afebrile and has no excessive muscle fasciculations.[40] | Leukogram, CSF analysis, IgM capture ELISA, plaque reduction neutralization test (PRNT),[39]absence of mosquito vectors.[27] |
| WEE | Systemically ill, pyrexia, abnormal motor function.[40] | Leukogram, ELISA, titres, virus isolation.[39] |
| EEE | Systemically ill, pyrexia, abnormal motor function[40], rapidly progressive.[39] | Leukogram, CSF analysis, ELISA, titres, virus isolation.[39] |
| VEE | Systemically ill, pyrexia. | Leukogram, IgM ELISA[41] |
| Equine herpesvirus-1 myeloencephalopathy | Sudden onset and early stabilization of neurological signs, multiple horses affected, recent fever, respiratory disease, abortion.[42] Dysuria not often seen in EPM.[4] | CSF analysis, buffy coat, nasal swab PCR.[27][39] |
| Rabies | Rapid progression[43], behavioural alterations, depression, seizure, coma.[40] | Post-mortem fluorescent antibody testing of brain required for definitive diagnosis.[43] |
| Polyneuritis equi (previously cauda equina neuritis) | Cranial nerve deficits are peripheral with no change in attitude.[44] | Western blot analysis of CSF.[45] |
| Equine degenerative myeloencephalopathy | Symmetrical signs.[46] | May get increased CSF creatinine kinase (CK)[47] and reduced serum Vitamin E concentrations but these are unreliable for ante mortem diagnosis.[46] |
| Verminous encephalomyelitis | Acute onset. | CSF analysis.[48] |
| Bacterial meningoencephalitis | Stiff neck.[1] | CSF analysis and culture. NB: CSF collection contraindicated if clinical signs suggest high intracranial pressure |
| CNS abscessation due to 'bastard strangles'[4] | History of Streptococcus equi subsp. equi infection.[49] | CSF analysis (severe, suppurative inflammation), culture of CSF.[49] |
| Spinal trauma[1] | History (usually acute onset neurological signs), usually solitary lesion localised by neurological exam.[50] | Radiography, myelography, CT, MRI, nuclear scintigraphy, ultrasound, CSF analysis, nerve conduction velocities, EMG, transcranial magnetic stimulation.[51] |
| Occipito-atlanto-axial malformation (OAAM) | Deficits develop before 6mths in Arabian horse.[52] | Radiography.[39] |
| Spinal tumor | Signs can usually be localized to one region of the CNS. | CT, MRI. Definitive diagnosis requires cytology, biopsy, histopathology or CSF analysis.[53] |
| Sorghum cystitis/ataxia[1] | Posterior ataxia or paresis, cystitis, history of grazing Sorghum species[54] | Demonstration of cystitis or pyelonephritis by laboratory methods, but not specific.[54] |
NB: EPM has been seen concurrently with equine motor neuron disease in a mule[55]
Pathology
Widespread lesions of the CNS are typically observed in horses.[4]
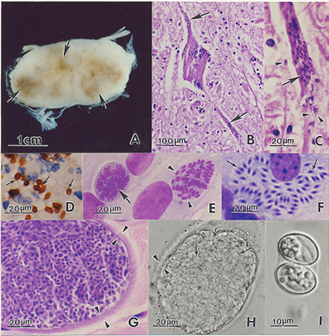
Gross exam
Lesions may be up to several centimetres across.[4] They range from mild discolouration to multifocal areas of haemorrhage and/or malacia[26] of the brain, spinal cord and less commonly, peripheral nerves.[4]
Histopathology
Microscopically, both grey and white matter may be affected with focal to diffuse areas of nonsuppurative inflammation, necrosis and neuronal destruction. Perivascular infiltrates comprise lymphocytes, macrophages, plasma cells, giant cells, eosinophils and gitter cells.[4] In around 25% of cases, schizonts or merozoites may be found in the neuronal cytoplasm.[26] Less frequently, protozoa parasitize intravascular and tissue neutrophils and eosinophils, capillary endothelial cells and myelinated axons[4][26]. Free merozoites may be seen in necrotic regions. If organisms are absent, the diagnosis relies on recognition of the inflammatory changes described above.[26]
Treatment
Antiprotozoals
The Food and Drug Administration (FDA) has approved four treatments for use in horses with EPM, but not all of these are commercially available:[27]
- Sulfadiazine and pyrimethamine combination, ('Rebalance™', Antiprotozoal Oral Suspension, IVX Animal Health): administered PO daily for a minimum of 90 days. Due to availability and ease of administration, some use an off-label regimen of trimethoprimsulfa tablets with pyrimethamine tablets. Pyrimethamine must be given at least 1 hr before or after hay is fed.[26] Mode of action: trimethoprim, sulfadiazine, and pyrimethamine all inhibit enzymes of folic acid synthesis. Efficacy: 61.5% improvement by one clinical grade.[56]Potential adverse effects: bone marrow suppression (mild anaemia, leucopenia, neutropenia, thrombocytopenia), fever, anorexia, depression, acute worsening of ataxia and altered reproductive performance in stallions[57], congenital defects[58]and abortion. Folic acid deficiency may also cause gastrointestinal disturbances such as glossitis.[59]Blood dyscrazias are typically self-limiting and resolve on withdrawal of treatment.[4] Feeding high quantities of green forage should reduce the risk of anaemia after prolonged treatment. [26]
- Ponazuril (Marquis®, Bayer Animal Health): PO daily for 28 days, use in pregnant animals is off-label. Mode of action: ponazuril is a triazinetrione that targets the “apicoplast” organelle and inhibits the respiratory chain. Efficacy: well absorbed PO, achieves steady state therapeutic concentration in CSF within 3 days[60]clinical response within 10 days, 60% improvement by at least one clinical grade, 8% relapse within 90 days of stopping treatment.[61] Potential adverse effects: none in a multi-centre field study[27], no systemic toxicity even at high doses.[62]However, the manufacturer reports signs that may have been related to treatment including blisters on the nose and mouth, skin rash or hives, loose stools, mild colic, and a seizure.[27]
- Diclazuril: PO, daily for 28 days, approved by FDA for use as top-dress tablet but not commercially available. Mode of action: chemically similar to ponazuril but mechanism of action unknown. Efficacy: one study reported clinical improvement in 58% of cases.[56] Potential adverse effects: none found in one efficacy study.[4] Reported problems in a multi-centre field study included worsening neurologic status and laminitis but these were not proven to be related to treatment.[27]
- Nitazoxanide, NTZ ('Navigator®', Idexx Pharmaceuticals): no longer commercially available in the US. Mode of action: a member of the 5-nitrothiazole class of antiparasitics that inhibits the pyruvate:ferredoxin oxidoreductase (PFOR) enzyme dependent electron transfer reaction essential for anaerobic energy metabolism.[27] Efficacy: 60% success rate in an FDA-regulated study.[56] Potential adverse effects: adverse effects and death at high doses[56], fever, anorexia, diarrhoea, lethargy, depression and laminitis recorded at lower doses. Toxic signs usally resolve upon cessation of treatment.[4] Caution: 'administration of nitazoxanide can disrupt the normal microbial flora of the gastrointestinal tract leading to enterocolitis. Deaths due to enterocolitis have been observed while administering the recommended dose in field studies.[27]
Prolonged, off-license treatment is often instigated after 1 month, based on repeated clinical examination. Even successfully treated cases may remain immunoblot positive for long periods, thus aiming for seronegativity is unrealistic.[4] A lack of response to treatment suggests that the diagnosis should be re-assessed. Another month's worth of the same treatment is recommended for partial responders, with switching to a different chemical class if this fails. The efficacy of currently approved antiprotozoals against N.hughesi is unknown.[4]
Ancillary medication
- NSAIDs: DMSO IV as 10% solution, thought to reduce CSF pressure and improve clinical status. Recommended for severe cases of EPM or to avoid worsening inflammation that may be induced by parasite kill.[4] Caution: DMSO may cause intravascular haemolysis.[1]
- Corticosteroids: a short course of dexamethasone may be beneficial whilst waiting for antiprotozoals to take effect. However, use is controversial because cell-mediated immunity is required to control parasites[1] and stress is a proposed risk factor for EPM.[4]
- Immunomodulators: Levamisole influences T-cell mediated immunity and enhances phagocytosis. Parapox ovis virus (PPOV) immunomodulator (Zylexis, Pfizer Animal Health, Kalamazoo, Mich). This vaccine has been shown to upregulate the secretion of cytokines including IFN-γ in several species.[63]IFN-γ is thought to be essential for the clearance of S.neurona, thus PPOV may be useful in EPM.[4]
- Multiple vitamin B supplement.[1]
Supportive management
Box rest with deep bedding and good footing or turn out in a flat, grassy field. Ensure all obstacles are removed and avoid turning out ataxic animals with dominant herd mates. Recumbent horses will require dedicated support and a sling if available.[4]
Prognosis
Depends on duration and severity of neurological signs[3] but clinical resolution is more likely if the condition is diagnosed and treated early.[2] With standard therapy, there is a recovery rate of around 25% and an improvement in 60-75% of cases.[56] A good prognosis might be expected if there is a response to treatment within two weeks. The prognosis will be guarded to poor[1] for a horse with severe, irreversible neuronal damage.
Prevention
Prophylaxis
A killed vaccine, developed using S.neurona merozoites, was conditionally licensed for use in horses.[64] The vaccine proved to be ineffective in the prevention of EPM and has since been removed from the market.[4] There is evidence to suggest that the antiprotozoal, ponazuril, may be useful prophylactically to reduce the incidence and severity of clinical signs.[65] Implementing such a regime prior to and during stressful events may be beneficial, although the cost is likely to be prohibitive.[4]Protocols involving intermittent administration of ponazuril may also show promise in the prevention of EPM.[66]
Control
Control of EPM is challenging because there are a variety of intermediate hosts for S.neurona and this parasite is very widely distributed.[4] The definitive host, the opossum, is also a scavenger and will consume road-kill, including species that are putative intermediate hosts for S.neurona.[64] A number of control measures are recommended:
- Deny wildlife access to feed (use rodent-proof containers, protect forages in enclosed facilities,[4] remove fallen fruit and bird feeders)[26]
- Prevent access of opossums to horse-feeding areas
- Remove carcasses from roads and property (especially those of skunks, raccoons, armadillos and cats which may act as intermediate hosts)[64]
- Opossums can be trapped and relocated[26]
- Monitor high-risk horses closely to help detect EPM early[4]
| Equine Protozoal Myeloencephalitis Learning Resources | |
|---|---|
 Search for recent publications via CAB Abstract (CABI log in required) |
Equine protozoal myeloencephalitis |
 Full text articles available from CAB Abstract (CABI log in required) |
Evidence-based review of diagnosis and treatment of Sarcocystis neurona infection (equine protozoal myeloencephalitis). Johnson, A. L.; White, N., II; American Association of Equine Practitioners (AAEP), Lexington, USA, Proceedings of the 55th Annual Convention of the American Association of Equine Practitioners, Las Vegas, Nevada, USA, 5-9 December 2009, 2009, pp 172-176, 27 ref. |
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 Pasquini, C, Pasquini, S, Woods, P (2005) Guide to Equine Clinics Volume 1: Equine Medicine (Third edition), SUDZ Publishing, 245-250. Cite error: Invalid
<ref>tag; name "Pasq" defined multiple times with different content Cite error: Invalid<ref>tag; name "Pasq" defined multiple times with different content Cite error: Invalid<ref>tag; name "Pasq" defined multiple times with different content Cite error: Invalid<ref>tag; name "Pasq" defined multiple times with different content Cite error: Invalid<ref>tag; name "Pasq" defined multiple times with different content Cite error: Invalid<ref>tag; name "Pasq" defined multiple times with different content Cite error: Invalid<ref>tag; name "Pasq" defined multiple times with different content Cite error: Invalid<ref>tag; name "Pasq" defined multiple times with different content Cite error: Invalid<ref>tag; name "Pasq" defined multiple times with different content Cite error: Invalid<ref>tag; name "Pasq" defined multiple times with different content Cite error: Invalid<ref>tag; name "Pasq" defined multiple times with different content Cite error: Invalid<ref>tag; name "Pasq" defined multiple times with different content Cite error: Invalid<ref>tag; name "Pasq" defined multiple times with different content Cite error: Invalid<ref>tag; name "Pasq" defined multiple times with different content - ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Gray, L.C, Magdesian, K.G, Sturges, B.K, Madigan, J.E (2001) Suspected protozoal myeloencephalitis in a two-month-old colt. Vet Rec, 149:269-273. Cite error: Invalid
<ref>tag; name "EPM8" defined multiple times with different content - ↑ 3.0 3.1 3.2 3.3 Vatistas, N, Mayhew, J (1995) Differential diagnosis of polyneuritis equi. In Practice, Jan, 26-29.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 4.19 4.20 4.21 4.22 4.23 4.24 4.25 4.26 4.27 4.28 4.29 4.30 4.31 4.32 4.33 4.34 4.35 4.36 4.37 4.38 4.39 4.40 4.41 Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Dubey, J.P, Lindsay, D.S, Saville, W.J, Reed, S.M, Granstrom, D.E, Speer, C.A (2001)A review of Sarcocystis neurona and equine protozoal myeloencephalitis (EPM). Vet Parasitol, 95:89-131. In: Pusterla, N, Wilson, W.D, Conrad, P.A, Barr, B.C, Ferraro, G.L, Daft, B.M, Leutenegger, C.M (2006) Cytokine gene signatures in neural tissue of horses with equine protozoal myeloencephalitis or equine herpes type 1 myeloencephalopathy. Vet Rec, Sep 9:Papers & Articles.
- ↑ Wobeser, B.K, Godson, D.L, Rejmanek, D, Dowling, P (2009) Equine protozoal myeloencephalitis caused by Neospora hughesi in an adult horse in Saskatchewan. Can Vet J, 50(8):851-3.
- ↑ Reed, S.M, Granstrom, D, Rivas, L.J, Saville, W.A, Moore, B.R, Mitten, L.A (1994) Results of cerebrospinal fluid analysis in 119 horses testing positive to the Western blot test on both serum and CSF to equine protozoal encephalomyelitis. In Proc Am Assoc Equine Pract, Vancouver BC, AEEP, Lexington, KY, p199. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Pitel, P.H, Pronost, S, Gargala, G, Anrioud, D, Toquet, M-P, Foucher, N, Collobert-Laugier, C, Fortier, G, Ballet, J-J (2002) Detection of Sarcocystis neurona antibodies in French horses with neurological signs, Int J Parasitol, 32:481-485. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Goehring, L.S (2001) Sloet van Oldruitenborgh-Oosterbaan MM: Equine protozoal myeloencephalitis in the Netherlands? An overview, Tijdschr Diergeneeskd, 126:346-351. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Saville, W.J.A, Reed, S.M, Granstrom, D.E, Morley, P.S (1997) Some epidemiologic aspects of equine protozoal myeloencephalitis. Proceedings of the Annual Convention of the AAEP, 43:6-7.
- ↑ 11.0 11.1 NAHMS (2000): Equine protozoal myeloencephalitis in the US, Ft Collins, CO, USDA:APHIS:VS, CEAH, National Animal Health Monitoring System. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ MacKay, R.J, Davis, S.W, Dubey, J.P (1992) Equine protozoal myeloencephalitis, Compend Contin Educ Pract Vet, 14:1359-1367. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Fayer, R, Mayhew, I.G, Baird, J.D, Dill, S.G, Foreman, J.H, Fox, J.C, Higgins, R.J Higgins, Reed, S.M, Ruoff, W.W, Sweeney, R.W, Tuttle, P (1990) Epidemiology of equine protozoal myeloencephalitis in North America based on histologically confirmed cases, J Vet Intern Med, 4:54-57. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Boy, M.G, Galligan, D.T, Divers, T.J (1990) Protozoal encephalomyelitis in horses: 82 cases (1972-1986), J Am Vet Med Assoc, 196:632-634. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Saville, W.J.A, Reed, S.M, Morley, P.S (1999) Examination of risk factors for equine protozoal myeloencephalitis. Proceedings of the Annual Convention of the AAEP, 45:48-49.
- ↑ Tillotson, K, McCue, P.M, Granstrom, D.E, Dargatz, D.A, Smith, M.O, Traub-Dargatz, J.L (1999) Seroprevalence of antibodies to Sarcocystis neurona in horses residing in northern Colorado, J Equine Vet Sci, 19:122-126. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Sofaly, C.D, Reed, S.M, Gordon, J.C, Dubey, J.P, Oglesbee, M, Njoku, D, Grover, C, Saville, W.J.A (2002) Experimental induction of equine protozoal myeloencephalitis (EPM) in the horse: effect of Sarcocystis neurona sporocyst inoculation dose on the development of clinical neurological disease, J Parasitol, 88:1164-1170. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Saville, W.J, Reed, S.M, Morley, P.S, Granstrom, D.E, Kohn, C.W, Hinchcliff, K.W, Wittum, T.E (2000) Analysis of risk factors for the development of equine protozoal myeloencephalitis in horses. J Am Vet Med Assoc, 217:1174-1180. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Njoku, C.J, Saville, W.J, Reed, S.M, Oglesbee, M.J, Rajala-Schultz, P.J, Stich, R.W (2002) Reduced levels of nitric oxide metabolites in cerebrospinal fluid are associated with equine protozoal myeloencephalitis, Clin Diagn Lab Immunol, 9:605-610. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Lindsay, D.S, Mitchell, S.M, Yang, J, Dubey, J.P, Gogal, R.M, Jr, Witonsky, S.G (2006) Penetration of equine leukocytes by merozoites of Sarcocystis neurona. Vet Parasitol, 15:138(3-4):371-6
- ↑ Pusterla, N, Wilson, W.D, Conrad, P.A, Barr, B.C, Ferraro, G.L, Daft, B.M, Leutenegger, C.M (2006) Cytokine gene signatures in neural tissue of horses with equine protozoal myeloencephalitis or equine herpes type 1 myeloencephalopathy, Vet Rec, 159:341-346.
- ↑ Spencer, J.A, Ellison, S.E, Guarino, A.J, Blagburn, B.L (2004) Cell-mediated immune responses in horses with equine protozoal myeloencephalitis. J Parasitol, 90(2):428-30.
- ↑ Yang, J, Ellison, S, Gogal, R, Norton, H, Lindsay, D.S, Andrews, F, Ward, D, Witonsky, S (2006) Immune response to Sarcocystis neurona infection in naturally infected horses with equine protozoal myeloencephalitis. Vet Parasitol, 138(3-4):200-10.
- ↑ Moore, L.A, Johnson, P.J, Messer, N.T, Kline, K.L, Crump, L.M, Knibb, J.R (1997) Management of headshaking in three horses by treatment for protozoal myeloencephalitis Vet Rec 141:264-267.
- ↑ Dunigan, C.E, Oglesbee, M.J, Podell, M 'et al.' (1995) Seizure activity associated with equine protozoal myeloencephalitis, Prog Vet Neurol, 6:50-54. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 26.6 26.7 26.8 26.9 Merck & Co (2008) The Merck Veterinary Manual (Eighth Edition), Merial
- ↑ 27.00 27.01 27.02 27.03 27.04 27.05 27.06 27.07 27.08 27.09 27.10 27.11 27.12 27.13 Johnson, A.L (2009) Evidence-based review of diagnosis and treatment of Sarcocystis neurona infection (Equine Protozoal Myeloencephalitis). Proceedings of the Annual Convention of the AAEP - Las Vegas, NV, USA, 55:172-176.
- ↑ 28.0 28.1 28.2 Johnson, A.L (2008) Evidence-based clinical question: which is the most sensitive and specific commercial test to diagnose Sarcocystis neurona infection (equine protozoal myeloencephalitis) in horses?, Equine Vet Educ, 20(3):166-168.
- ↑ Furr, M (2002) Antigen-specific antibodies in cerebrospinal fluid after intramuscular injection of ovalbumin in horses, J Vet Intern Med, 16:588-592. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Duarte, P.C, Daft, B.M, Conrad, P.A, Packham, A.E, Gardner, I.A (2003) Comparison of a serum indirect fluorescent antibody test with two Western blot tests for the diagnosis of equine protozoal myeloencephalitis, J Vet Diagn Invest, 15:8-13. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Granstrom, D.E (1995) Equine protozoal myeloencephalitis testing: review of 1993 and 1994. Proc Annu Conv Am Assoc Equine Prac, 41:218-219. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Finno, C.J, Packham, A.E, Wilson, W.D, et al. (2007) Effects of blood contamination of cerebrospinal fluid on results of indirect fluorescent antibody tests for detection of antibodies against Sarcocystis neurona and Neospora hughesi. J Vet Diag Invest, 19:286–289. In: Johnson, A.L (2009) Evidence-based review of diagnosis and treatment of Sarcocystis neurona infection (Equine Protozoal Myeloencephalitis). Proceedings of the Annual Convention of the AAEP - Las Vegas, NV, USA, 55:172-176.
- ↑ Howe, D, Gaji, R, Marsh, A (2008) Strains of S.neurona exhibit differences in their surface antigens, including the absence of the major surface antigen SnSAG1. Int J Parasitol, 38:623-631. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Crowdus, C.A, Marsh, A.E, Saville, W.J, et al. (2008) SnSAG5 is an alternative surface antigen of Sarcocystis neurona strains that is mutually exclusive to SnSAG1. Vet Parasitol, 158:36–43. In: Johnson, A.L (2009) Evidence-based review of diagnosis and treatment of Sarcocystis neurona infection (Equine Protozoal Myeloencephalitis). Proceedings of the Annual Convention of the AAEP - Las Vegas, NV, USA, 55:172-176.
- ↑ Bentz, B.G, Dirikolu, L, Carter, W.G, Saville, W.J.A, Williams, N.M, Bernard, W.V, Wulff-Strobel, C, Baker, C.B, McCrillis, S, Reed, S, Harkins, Granstrom, D.E, Tobin, T (2000) Special Article: Diclazuril and equine protozoal myeloencephalitis (EPM): a clinical report. Equine Vet Educ, 12(4):195-200.
- ↑ Eastman, E, Furr, M, McKenzie, H, Saville, W.J, Dubey, J.P (2005) Early diagnosis of Sarcocystis neurona infection using bloodgene expression biomarkers. In: 51st Annual Convention of the American Association of Equine Practitioners - AAEP, Seattle, WA, USA.
- ↑ Mayhew, I.G, deLahunta, A, Whitlock, R.H, Krook, L, Tasker, J.B (1978) Spinal cord disease in the horse, Cornell Vet, 68(Suppl 8):110-120. In: Hahn, C.N (2010) Cervical Vertebral Malformation in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ 38.0 38.1 Hahn, C.N (2010) Cervical Vertebral Malformation in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ 39.0 39.1 39.2 39.3 39.4 39.5 39.6 Seino, K.K (2010) Spinal Ataxia in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 3.
- ↑ 40.0 40.1 40.2 40.3 Long, M.T (2010) Flavivirus Encephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Bertone, J.J (2010) Viral Encephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Wilson, W.D, Pusterla, N (2010) Equine Herpesvirus-1 Myeloencephalopathy in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ 43.0 43.1 Sommardahl, C.S (2010) Rabies in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Scaratt, W.K, Jortner, B.S (1985) Neuritis of the cauda equina in a yearling filly. Compend Contin Educ Pract Vet, 7:S197-S202. In: Saville, W.J (2010) Polyneuritis equi in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Granstrom, D.E, Dubey, J.P, Giles, R.C (1994) Equine protozoal myeloencephalitis: biology and epidemiology. In Nakajima, H, Plowright, W, editors: Refereed Proceedings, Newmarket, England, R & W Publications. In: Saville, W.J (2010) Polyneuritis equi in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ 46.0 46.1 Nout, Y.S (2010) Equine Degenerative Myeloencephalopathy in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Mayhew, I.G, deLahunta, A, Whitlock, R.H, Krook, L, Tasker, J.B (1978) Spinal cord disease in the horse, Cornell Vet, 68(Suppl 8):1-207. In: Nout, Y.S (2010) Equine Degenerative Myeloencephalopathy in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Jose-Cunilleras, E (2010) Verminous Encephalomyelitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ 49.0 49.1 Byrne, B. A (2010) Diseases of the Cerebellum in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Smith, P.M, Jeffery, N.D (2005) Spinal shock - comparative aspects and clinical relevance. J Vet Intern Med, 19:788-793. In: Nout, Y.S (2010) Central Nervous System Trauma in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Nout, Y.S (2010) Central Nervous System Trauma in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Watson, A.G, Mayhew, I.G (1986) Familial congenital occipitoatlantoaxial malformation (OAAM) in the Arabian horse. Spine, 11:334-339. In: Seino, K.K (2010) Spinal Ataxia in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 3.
- ↑ Sellon, D.C (2010) Miscellaneous Neurologic Disorders in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ 54.0 54.1 Talcott, P (2010) Toxicoses causing signs relating to the urinary system in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 22.
- ↑ Finno, C.J, Eaton, J.S, Aleman, M, Hollingsworth, S.R (2010) Equine protozoal myeloencephalitis due to Neospora hughesi and equine motor neuron disease in a mule. Vet Ophthalmol, 13(4):259-65.
- ↑ 56.0 56.1 56.2 56.3 56.4 MacKay, R.J (2006) Equine protozoa myeloencephalitis: treatment, prognosis and prevention. Clin Tech Equine Pract, 5:9-16. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Bedford, S.J, McDonnell, S.M (1999) Measurements of reproductive function in stallions treated with trimethoprim-sulfamethoxazole and pyrimethamine. J Am Vet Med Assoc, 215:1317–1319. In: Johnson, A.L (2009) Evidence-based review of diagnosis and treatment of Sarcocystis neurona infection (Equine Protozoal Myeloencephalitis). Proceedings of the Annual Convention of the AAEP - Las Vegas, NV, USA, 55:172-176.
- ↑ Toribio, R.E, Bain, F.T, Mrad, D.R, Messer, N.T, Sellers, R.S, Hinchcliff, K.W (1998) Congenital defects in newborn foals of mares treated for equine protozoal myeloencephalitis during pregnancy. J Am Vet Med Assoc, 212:697–701. In: Johnson, A.L (2009) Evidence-based review of diagnosis and treatment of Sarcocystis neurona infection (Equine Protozoal Myeloencephalitis). Proceedings of the Annual Convention of the AAEP - Las Vegas, NV, USA, 55:172-176.
- ↑ Piercy, R.J, Hinchcliff, K.W, Reed, S.M (2002) Folate deficiency during treatment with orally administered folic acid, sulphadiazine and pyrimethamine in a horse with suspected equine protozoal myeloencephalitis (EPM). Equine Vet J, 34:311–316. In: Johnson, A.L (2009) Evidence-based review of diagnosis and treatment of Sarcocystis neurona infection (Equine Protozoal Myeloencephalitis). Proceedings of the Annual Convention of the AAEP - Las Vegas, NV, USA, 55:172-176.
- ↑ Furr, M, Kennedy, T (2001) Cerebrospinal fluid and serum concentrations of ponazuril in horses. Vet Ther, 2:232-237. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Furr, M, Kennedy, T, MacKay, R, Reed, S, Andrews, F, Bernard, B, Bain, F, Byars, D (2001) Efficacy of ponazuril 15% oral paste as a treatment for equine protozoal myeloencephalitis. J Vet Ther, 2:215-222. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Kennedy, T, Campbell, J, Selzer, V (2001) Safety of ponazuril 15% oral paste in horses. Vet Ther, 2:223-231. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Frieb, A, Siegling, A, Friederichs, S, Volk, H-D, Weber, O (2004) Effects of inactivated parapoxvirus ovis (orf virus) on human peripheral immune cells: induction of cytokine secretion in monocytes and Th1-like cells. J Virol, 78:9400-9411. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ 64.0 64.1 64.2 Saville, W.J.A, Reed, S.M, Dubey, J.P (2002) Prevention of equine protozoal myeloencephalitis(EPM). Proceedings of the Annual Convention of the AAEP, 48:181-185.
- ↑ Furr, M, MacKenzie, H, Dubey, J.P (2006) Pretreatment of horses with ponazuril limits infection and neurologic signs resulting from S.neurona. J Parasitol, 92:637-643. In: Furr, M (2010) Equine protozoal myeloencephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 12.
- ↑ Mackay, R.J, Tanhauser, S.T, Gillis, K.D, Mayhew, I.G, Kennedy, T.J (2008) Effect of intermittent oral administration of ponazuril on experimental Sarcocystis neurona infection of horses. Am J Vet Res, 69(3):396-402.
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
Error in widget FBRecommend: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt662b3c137d9801_54492776 Error in widget google+: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt662b3c1380c015_99492694 Error in widget TwitterTweet: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt662b3c138485f8_86306864
|
| WikiVet® Introduction - Help WikiVet - Report a Problem |