Difference between revisions of "Gastric Ulceration - Horse"
| Line 41: | Line 41: | ||
==Pathophysiology== | ==Pathophysiology== | ||
| + | The cause of EGUS is multifactorial (Jonssen 2006) Differences in the aetiology of ulcers in different locations of the stomach. (Luthersson et al 2009) | ||
| + | |||
| + | Hydrochloric, lactic acid and volatile fatty acids | ||
| + | Hydrochloric acid (HCl) and a sustained gastric pH<4.0 are probably the most important cause of gastric ulcers. Recently, other acids (volatile fatty acids [VFAs], lactic acid and bile acids) have been shown to act synergistically with HCl to cause changes in nonglandular mucosal bioelectric properties, which are the first indication of acid injury. VFAs and lactic acid are byproducts of bacterial fermentation of sugars in concentrate diets. | ||
| + | Ulcers are most prevalent in the nonglandular mucosa because this area lacks resistance to acid injury. HCl induces injury in this region by damaging the outer cell barrier, followed later by diffusion into the squamous cells of the stratum spinosum resulting in inhibition of cellular sodium transport, cell swelling and eventual ulceration (Argenzio and Eismann 1987; Nadeau et al. 2003a,b). | ||
| + | Whereas, VFAs (acetic, propionic, butyric and valeric acids), because of their lipid solubility, induce damage by rapidly diffusing into the squamous mucosal cells of the stratum spinosum layer and immediately inhibit sodium transport which results in cell swelling and ulceration. Squamous mucosal cells are susceptible to HCl and VFA injury in pH, dose and time dependent manner (Andrews et al. 2006). | ||
| + | Lactic acid has a similar chemical structure to VFAs. But lactic acid (pH 1.5 and 40 mmol/l) exposed to the nonglandular mucosa increased tissue permeability, as indicated by increased transepithelial conductance (Andrews et al. 2008). | ||
| + | Furthermore, HCl and VFAs have been shown to cause disruption in bioelectric properties and barrier function of the NG mucosa of horses in an in vitro Ussing chamber system (Widenhouse et al. 2002; Nadeau et al. 2003a,b; Andrews et al. 2006). The proposed mechanism by which VFAs cause acid injury is as follows: at low pH (≤4.0), VFAs remain undisassociated (nonionic) and are highly lipid soluble. By penetrating the NG mucosal cells and acidifying cellular contents they disrupt cellular sodium transport, leading to cell swelling, cell death and ulceration (Argenzio and Eisemann 1996; Nadeau | ||
| + | et al. 2003a,b). | ||
| + | Lactic acid (LA), commonly found in the stomach of horses after eating, has a chemical structure and low pKa (3.8) similar to acetic acid, but LA is stronger. In previous studies, LA concentrations in the stomach were high (Wolter and Chaabouni 1979; Al Jassim 2006), compared to other regions of the digestive tract. Stomach LA is probably produced by resident acid-tolerant bacteria, such as Lactobacillus and Streptococcus spp., which have been found in abundance in the equine stomach (Al Jassim | ||
| + | et al. 2005; Varloud et al. 2007). Also, a recent study showed a3-fold increase in post prandial L-/D-lactate concentration (Varloud et al. 2007). Therefore, LA exposure to NG mucosa of horses in an acidic environment would be expected to cause similar acid injury to that caused by other VFAs. | ||
| + | Lactic acid, produced in the stomach of horses fed a high-grain diet, does not significantly alter sodium transport or permeability in equine NG mucosal tissue. Lactic acid may require a longer exposure time or may need the presence of other VFAs in HCl to cause gastric ulcers.(Andrews 2008) | ||
| + | |||
| + | Helicobacter spp. and other bacteria | ||
| + | Recently a new enterohepatic Helicobacter species, Helicobacter equorum, was isolated from faecal samples of 2 clinically healthy horses (Fox 2002). Also, Helicobacter equorum DNA was demonstrated in the faeces of 2/7 (28.6%) foals aged <1 month and 40/59 (67.8%) foals aged 1–6 months (Moyaert et al. 2009). Furthermore, Helicobacterlike DNA was detected in the stomachs of 10 Thoroughbred horses in Venezuela (Contreras et al. 2007). Furthermore, 10/11 of the horses infected with Helicobacter had either gastric ulcers or gastritis or both pathologies. However, 39% of the horses in that study did not have gastric lesions, so multiple causes are likely. | ||
| + | Bacteria, including E. coli, were cultured from the stomach of horses (Al Jassim et al. 2006). Bacterial colonisation of gastric ulcers in the stomach of horses may delay ulcer healing and in this case treatment with antibiotics may be indicated.(Nadeau 2009) | ||
| + | |||
| + | The pathophysiology of gastric ulcer formation involves an imbalance between aggressive (HCl, pepsin) and defensive factors. In the nonglandular region, intercellular tight junctions and intracellular buffering systems act as barriers, while the glandular region is protected by a 200 μmol/l thick mucus layer into which bicarbonate ions are secreted (Murray 1992b). Adequate mucosal blood flow and mucus secretion is maintained by appropriate prostaglandin release (Morrissey et al. 2008). Epidermal growth factor has also been found to contribute to the healthy maintenance and repair of equine gastric squamous epithelium (Jeffrey et al. 2001). Any disruption in the chain of these events may act to promote damage and ulceration. | ||
| + | (Martineau 2009) | ||
| + | |||
| + | Major intrinsic factors promoting ulcer formation include HCl, bile acids, pepsin, HCl is predominant. Intrinsic factors protect against ulcers: mucus-bicarbonate layer, maintenance of adequate mucosal blood flow, mucosal PG E2 and EGF production, gastrodudodenal motility. Serum Abs vs H.pylori are common in post-suckling foals and their dams (32) Other extrinsic ulcerogenic factors that may be important in horses include: NSAIDs, stress, changes in diet, GI disorders, esp those resulting in delayed gastric emptying (1). | ||
| + | Pathophysiology of squamous mucosal ulceration appears similar to gastrooesophageal relfuc disease (GERD) in human beings and ulceration of non-glandular mucosa in pigs. XS acid exposure is predmoninant mechanism but details unclear (34). HCl is secreted by parietal cells in gastric glands via H+K+-ATPase) pump on luminal side. Horses secrete acid continuously and measured pH from equine gastric contents is variable from less than 2 to greater than 6, depending on dietary state (fed vs fasted) (35,36). A protocol of repeated 24h periods of fasting and feeding(37) results in prolonged gastric acidity (pH <2) and because concurrent ranitdine reduces lesion severity, it supports the role of acid exposure in pathogenesis. | ||
| + | Predominant stimuli to HCl secretion are gastrin, histamine and Ach via vagus nerve (1). Gastrin is released by G cells within gastric mucosa, histamine by mast cells and ECL cells in gastric gland. Histamine binds to type 2 receptors on parietal cell membrane causing an increase in cAMP resulting in phosphorylation of enzymes that activate proton pump. Gastrin and Ach can act via calcium-mediated (Sanchez) | ||
| + | |||
| + | gastric acidity of a horse or foal is very high between periods of eating or nursing. Ulcers in the squamous mucosa result from increased exposure to hydrochloric acid, which can be secondary to prolonged periods of not eating or nursing, intensive exercise, or delayed gastric emptying. (Merck) | ||
| + | |||
| + | Am J Vet Res. 2010 May;71(5):592-6. | ||
| + | Expression of cyclooxygenase isoforms in ulcerated tissues of the nonglandular portion of the stomach in horses. | ||
| + | Rodrigues NL, Doré M, Doucet MY. | ||
| + | Increased expression of COX-2 in gastric ulcers of the squamous portion of the stomach in horses suggested a role for this enzyme in gastric ulcer healing. | ||
| + | |||
| + | Anatomy | ||
| + | Equine gastric ulcers occur in the squamous portion of the stomach in 75-80% of cases. | ||
| + | The squamous epithelium, which covers approximately one third of the stomach wall (Fig 21) and the oesophagus, is a relatively simple structure that consists of a tightly bound cornified superficial layer of cells that serves as a protective barrier. The squamous portion of the stomach lining has no absorptive or secretory function, leaving it more vulnerable to peptic injury. | ||
| + | The more complex glandular portion of the stomach contains mucus-secreting cells and gastric glands | ||
| + | (Fig 221, all of which respond to various stimuli and provide secretions that have a specific function. The gastric glands contain six predominant cell types (Table 3). | ||
| + | Physiology | ||
| + | The parietal cell secretes H+ via H+/K+ ATPase proton pump (Fig 19) located on its apical membrane. The K+ used by this proton pump and the C1- that combines with the H+ are secreted through the same apical membrane via ion-specific channels. The resultant HCl moves up through the gastric gland and into the gastric lumen, lowering the pH of the gastric contents. Acid and the enzyme pepsin exert peptic influences on digestion. The stomach has a number of protective mechanisms that prevent acid and peptic activity from causing inappropriate mucosal destruction. | ||
| + | Glandular mucosal defence mechanisms | ||
| + | Two important substances involved in gastric glandular mucosal protection are epidermal growth factors (EGFs) and prostaglandin E2 (PGE2). Epidermal growth factors are found in salivary gland secretions and promote DNA synthesis and proliferation of gastric mucosal cells. They also play a role in prostaglandin synthesis. The PGEz promotes numerous protective functions within the gastric mucosa which include: | ||
| + | Suppression of HC1 secretion | ||
| + | Mucus secretion | ||
| + | Bicarbonate secretion | ||
| + | Epithelial restitution mechanisms (maintain tight junctions) | ||
| + | Adequate mucosal blood supply. | ||
| + | Mucus, secreted by specialised mucous neck cells, is a viscous, hydrophobic glycoproteinaceous gel that adheres to the mucosa and resists acid and pepsin contact. The gel also acts as a lubricant that minimises mechanical damage by gastric contents. | ||
| + | Bicarbonate secretion by gastric mucosal cells is triggered by a response to luminal acid concentrations, mechanical irritation, and by release of endogenous prostaglandins. Bicarbonate trapped in the mucous barrier adhering to the stomach wall forms a pH gradient that allows a physiological pH at the mucosal surface and a pH similar to that of stomach acid at the luminal surface. | ||
| + | Prostaglandins have an important role in gastric mucosal protection, although their precise mechanism is vague. Prostaglandins inhibit acid secretion, promote mucosal blood flow (vasodilate), increase mucus and bicarbonate secretions, and support mucosal cell repair. | ||
| + | Treatment with nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or the administration of prostaglandin antibodies results in ulcer formation in various species, including horses. This effect can be blunted by administering exogenous prostaglandins. | ||
| + | Epithelial cell restitution is another important mechanism in the maintenance of gastric mucosal integrity. Epithelial cells act as a protective covering, counter shear forces that induce damage, and provide rapid restoration of damaged protective barriers. In the case of the gastric mucosa, epithelial injury induces a migration of adjacent cells to replace damaged cells. This occurs within minutes without need of new cell proliferation. Shear forces, induced by mixing of ingested material raking against the mucosal wall, cause cell damage that is normally countered by the process of epithelial restoration. | ||
| + | An abundant mucosal blood supply is required to provide the mucosa with the oxygen and nutrients necessary to produce the mucus-bicarbonate layer and to support the rapid turnover of epithelial cells. An adequate blood supply is also required to remove acid that has diffused through the mucous layer to the mucosa. Alterations in blood flow (i.e., shock, microvascular thrombosis) are highly correlated with mucosal erosions and gastric ulcers in man. Epidermal growth factors are found in salivary gland secretions and promote DNA synthesis and proliferation of gastric mucosal cells. They also play a role in prostaglandin synthesis and inhibit the parietal gland secretions of hydrochloric acid. | ||
| + | Squamous mucosal defence mechanisms | ||
| + | Many discussions surrounding equine squamous disease are extrapolated from human oesophageal disease and data from other species. In these species, the initial response of squamous tissues exposed to acid is to thicken. | ||
| + | Cell-to-cell junctions are closely adhered (‘tight junctions’), which ensures a weak acid barrier. Buffering also is a component of the squamous tissue defence scheme, but unlike the glandular portion, there is no external mucus-bicarbonate layer. Rather, squamous tissues buffer internally (within the cell) and use leucotrienes for defence. By contrast, glandular tissue relies on prostaglandins for adequate mucosal protection. | ||
| + | In summary, the squamous tissues of the stomach have a limited number of defence mechanisms that centre around acid repulsion and intracellular buffering. Once the acid penetrates these defences, it builds up within the cell layers and necrosis (cell death) leads to ulcer formation. | ||
| + | Gastric acidity and secretion | ||
| + | The horse secretes acid in a continuously variable pattern, such that acid secretion occurs in the absence of ingestion of feed. The mean pH of gastric fluid in horses withheld from feed for several hours has consistently been found to be 2.0 or less. However, periodic fluctuations, in which the pH reaches 6-7 for 5 to 15 min, are commonly seen. Whereas acid output reflects the magnitude of hydrochloric acid secretion, gastric acidity is determined by the pH of gastric secretions and/or contents. | ||
| + | The distinction between gastric acid output and pH is relevant, since administration of antisecretory medications may significantly decrease acid output but have minimal effect on gastric fluid pH in horses until the output becomes very small. | ||
| + | Few studies on the effect of feeding on gastric physiology have been performed in horses. Serum gastrin increase after feeding was more profound when grain was fed compared with feeding only hay. Horses with free access to hay had greater mean 24 h gastric pH than did horses withheld from feed for 24 h (3.4 * 0.9 vs. 1.9 * 0.5). | ||
| + | These latter results support the continuous acid secretion of the equine stomach and the buffering capacity of feed and bicarbonate-rich salivary secretions that are stimulated by feeding. In foals, milk has been demonstrated to have a marked buffering effect. | ||
| + | |||
| + | Extrapolating from other species, causes of glandular ulcer formation would include: | ||
| + | Bile reflux into the stomach | ||
| + | Hypotensiod shock (resulting in the diminution of mucosal blood flow) | ||
| + | Increased sympathetic tone (leading to a decrease in mucosal blood flow) | ||
| + | Severe disease states such as uraemia, infections, coagulopathies and conditions that result in the impairment of blood flow | ||
| + | Neurological imbalance, resulting in impairment of gastric motility (results in the accumulation of acids) | ||
| + | Micro-organisms | ||
| + | .(EGUC) | ||
| + | |||
| + | It has been proposed that the disease in a high performance horse is directly linked to unnatural feeding, exercise and management regimes that result in either increased acidity of gastric contents or increased exposure of vulnerable areas of the stomach to acid contents (Murray and Eichorn 1996; Berschneider et al. 1999;Nadeau et al. 2000; Lorenzo-Figueras et al. 2002; Bell et al. 2007). | ||
| + | For horses kept in a more natural environment with continuous grass feeding, as some of these were, the aetiology of disease is more puzzling.(Martineau 2009) | ||
| + | |||
| + | |||
| + | We conclude that combinations of bile salts and acid are more injurious to the stratified squamous gastric mucosa of the equine than acid alone. Concentrations of bile salts and acid sufficient to alter the electrolyte transport function of this mucosa can be found in the gastric contents of horses deprived of feed for as little as 14 h.(Berschneider 1999) | ||
| + | |||
| + | In the horse, the junction of the glandular and squamous epithelia is at the margo plicatus, analagous to the gastro-oesophageal junction in man. Therefore, gastric ulceration seen in the equine patient more closely resembles oesophagitis and oesophageal ulceration seen in man (Collier and Stoneham 1997). Severe training regimes may cause diversion of blood flow to muscle, decreasing mucosal blood flow leading to a fall in mucosal resistance.(Collier 1999) | ||
| + | |||
| + | Horses secrete gastric acid continuously, even when they are not eating (Campbell-Thompson and Merritt 1987). | ||
| + | Grains contain less buffering material than hay and also contribute an acid load to the system (Roby et al. 1987). | ||
| + | There also appears to be an effect of exercise on the stomach that is independent of feeding, as horses fed the same diet before and during training had higher gastrin levels during training (Furr et al. 1994). Whether this is a stress response or some other physiological effect is unknown, but it suggests that exercise itself may be a predisposing factor in the development of gastric ulcers. | ||
| + | Stress has been thought to contribute to gastric ulcers or at least to epigastric distress in man and severe stress, associated with illness, may cause ulcers in man and in foals, but only in the glandular portion of the stomach (Furr et al. 1992). (Orsini) | ||
| + | |||
| + | |||
| + | Foal sites of ulceration: | ||
| + | Healthy foals: squamous mucosa, margo plicatus, greater curvature, squamous epithelial desquamation | ||
| + | Sick foals: margo plicatus, lesser curvature and cardia, glandular mucosa, pylorus and duodenum | ||
| + | NOT associated with ''Helicobacter pylori'' and not typically associated with ''Gasterophilus'' | ||
| + | |||
| + | Foals: hx, cx, gastric reflux, +/- occult blood in faeces, | ||
| + | Foals - lesions mainly in glandular epithelium | ||
| + | Adults - margo plicatus and squamous epithelium | ||
==Risk Factors== | ==Risk Factors== | ||
Revision as of 15:00, 30 July 2010
| This article is still under construction. |
| Also known as: | Gastroduodenal ulceration Gastrointestinal ulceration |
| See also: | Gastric Ulceration - all species |
Description
The term 'Equine gastric ulcer syndrome (EGUS)' encompasses a number of disease complexes[1] associated with ulceration of the oesophageal, gastric or duodenal mucosa[2] in horses. When such damage is caused by acidic gastric juice, the defect is described as a 'peptic ulcer'.[2] Ulceration of either or both[3] regions of the gastric mucosa is one of the most important problems of the equine stomach as it may limit performance[4] and compromise welfare.[5] The non-glandular (proximal or orad) region of the equine stomach is lined by stratified squamous mucosa and a glandular mucosa lines the distal (aborad) portion. The two regions meet abruptly at the margo plicatus[6], adjacent to where most ulcers occur.[2] Damage to these regions occurs via differing pathophysiological routes and varies in severity from inflammation, to cellular death and sloughing causing disruption of the superficial mucosa (erosion), penetration of the submucosa down to the level of the lamina propria[2](ulceration), full thickness ulceration (perforation)[6] and potentially duodenal stricture.[7] The occult nature of the disease typically precludes the observation of clinical signs until severe ulceration has developed.[2]
Prevalence
The prevalence of equine gastric ulceration has increased over the last century.[8] In a retrospective study of 3715 Swedish horses, ulcers were most often found in the squamous mucosa along the margo plicatus, then the glandular body, proximal squamous mucosa and antrum.[8] For the squamous region, reported prevalences are:
- Racehorses 66-93%[9][10][11]
- Racehorses in active race training 80-93% (incidence 100%)[12][13]
- Show horses 58%[14]
- Ponies 78%[15]
- Endurance horses 67%[16]
- Western performance horses 40%[17]
- Thoroughbred broodmares (67-77%)[18]
- Nonracing performance horses (17% pre-competition, 56% post-competition)[19]
- Pleasure horses in full work ~ 60%[4]
- Pleasure, riding lessons, showing 37%[20]
- Foals ~25-57%[21][22][23], the incidence increases dramatically in foals with clinical signs, especially gastrointestinal signs.[2]
The prevalence and severity of ulcers increases with work intensity[7] and duration[24][25], thus racehorses in active training are more often affected[9] and in half of these, the lesions are moderate to severe.[7] In one study, all horses developed gastric ulcers within 2 weeks of entering simulated race training.[12] Lesions are thought to be chronically progressive during race training, but to regress during retirement.[9] Horses with signs of gastrointestinal distress also demonstrate an increased frequency and severity of ulcerative lesions.[2]EGUS prevalence is high in horses with bowel, liver and oesophageal lesions.[8] Among show horses, 82% of those with signs of abdominal discomfort had gastric ulcers[26] Around 30% of adult horses and about 50% of foals have mild gastric erosions which heal without treatment or clinical signs.[7] In 201 clinically normal horses in Denmark, 53% had EGUS with severity score >2 and older horses were more likely to have lesions in both regions of the stomach[27]
Signalment
EGUS develops in horses of all ages[6] but is most common in young horses in training and foals. Gastric ulceration is considered to be rare in horses at pasture.[28]
Pathophysiology
The cause of EGUS is multifactorial (Jonssen 2006) Differences in the aetiology of ulcers in different locations of the stomach. (Luthersson et al 2009)
Hydrochloric, lactic acid and volatile fatty acids Hydrochloric acid (HCl) and a sustained gastric pH<4.0 are probably the most important cause of gastric ulcers. Recently, other acids (volatile fatty acids [VFAs], lactic acid and bile acids) have been shown to act synergistically with HCl to cause changes in nonglandular mucosal bioelectric properties, which are the first indication of acid injury. VFAs and lactic acid are byproducts of bacterial fermentation of sugars in concentrate diets. Ulcers are most prevalent in the nonglandular mucosa because this area lacks resistance to acid injury. HCl induces injury in this region by damaging the outer cell barrier, followed later by diffusion into the squamous cells of the stratum spinosum resulting in inhibition of cellular sodium transport, cell swelling and eventual ulceration (Argenzio and Eismann 1987; Nadeau et al. 2003a,b). Whereas, VFAs (acetic, propionic, butyric and valeric acids), because of their lipid solubility, induce damage by rapidly diffusing into the squamous mucosal cells of the stratum spinosum layer and immediately inhibit sodium transport which results in cell swelling and ulceration. Squamous mucosal cells are susceptible to HCl and VFA injury in pH, dose and time dependent manner (Andrews et al. 2006). Lactic acid has a similar chemical structure to VFAs. But lactic acid (pH 1.5 and 40 mmol/l) exposed to the nonglandular mucosa increased tissue permeability, as indicated by increased transepithelial conductance (Andrews et al. 2008). Furthermore, HCl and VFAs have been shown to cause disruption in bioelectric properties and barrier function of the NG mucosa of horses in an in vitro Ussing chamber system (Widenhouse et al. 2002; Nadeau et al. 2003a,b; Andrews et al. 2006). The proposed mechanism by which VFAs cause acid injury is as follows: at low pH (≤4.0), VFAs remain undisassociated (nonionic) and are highly lipid soluble. By penetrating the NG mucosal cells and acidifying cellular contents they disrupt cellular sodium transport, leading to cell swelling, cell death and ulceration (Argenzio and Eisemann 1996; Nadeau et al. 2003a,b). Lactic acid (LA), commonly found in the stomach of horses after eating, has a chemical structure and low pKa (3.8) similar to acetic acid, but LA is stronger. In previous studies, LA concentrations in the stomach were high (Wolter and Chaabouni 1979; Al Jassim 2006), compared to other regions of the digestive tract. Stomach LA is probably produced by resident acid-tolerant bacteria, such as Lactobacillus and Streptococcus spp., which have been found in abundance in the equine stomach (Al Jassim et al. 2005; Varloud et al. 2007). Also, a recent study showed a3-fold increase in post prandial L-/D-lactate concentration (Varloud et al. 2007). Therefore, LA exposure to NG mucosa of horses in an acidic environment would be expected to cause similar acid injury to that caused by other VFAs.
Lactic acid, produced in the stomach of horses fed a high-grain diet, does not significantly alter sodium transport or permeability in equine NG mucosal tissue. Lactic acid may require a longer exposure time or may need the presence of other VFAs in HCl to cause gastric ulcers.(Andrews 2008)
Helicobacter spp. and other bacteria Recently a new enterohepatic Helicobacter species, Helicobacter equorum, was isolated from faecal samples of 2 clinically healthy horses (Fox 2002). Also, Helicobacter equorum DNA was demonstrated in the faeces of 2/7 (28.6%) foals aged <1 month and 40/59 (67.8%) foals aged 1–6 months (Moyaert et al. 2009). Furthermore, Helicobacterlike DNA was detected in the stomachs of 10 Thoroughbred horses in Venezuela (Contreras et al. 2007). Furthermore, 10/11 of the horses infected with Helicobacter had either gastric ulcers or gastritis or both pathologies. However, 39% of the horses in that study did not have gastric lesions, so multiple causes are likely. Bacteria, including E. coli, were cultured from the stomach of horses (Al Jassim et al. 2006). Bacterial colonisation of gastric ulcers in the stomach of horses may delay ulcer healing and in this case treatment with antibiotics may be indicated.(Nadeau 2009)
The pathophysiology of gastric ulcer formation involves an imbalance between aggressive (HCl, pepsin) and defensive factors. In the nonglandular region, intercellular tight junctions and intracellular buffering systems act as barriers, while the glandular region is protected by a 200 μmol/l thick mucus layer into which bicarbonate ions are secreted (Murray 1992b). Adequate mucosal blood flow and mucus secretion is maintained by appropriate prostaglandin release (Morrissey et al. 2008). Epidermal growth factor has also been found to contribute to the healthy maintenance and repair of equine gastric squamous epithelium (Jeffrey et al. 2001). Any disruption in the chain of these events may act to promote damage and ulceration. (Martineau 2009)
Major intrinsic factors promoting ulcer formation include HCl, bile acids, pepsin, HCl is predominant. Intrinsic factors protect against ulcers: mucus-bicarbonate layer, maintenance of adequate mucosal blood flow, mucosal PG E2 and EGF production, gastrodudodenal motility. Serum Abs vs H.pylori are common in post-suckling foals and their dams (32) Other extrinsic ulcerogenic factors that may be important in horses include: NSAIDs, stress, changes in diet, GI disorders, esp those resulting in delayed gastric emptying (1). Pathophysiology of squamous mucosal ulceration appears similar to gastrooesophageal relfuc disease (GERD) in human beings and ulceration of non-glandular mucosa in pigs. XS acid exposure is predmoninant mechanism but details unclear (34). HCl is secreted by parietal cells in gastric glands via H+K+-ATPase) pump on luminal side. Horses secrete acid continuously and measured pH from equine gastric contents is variable from less than 2 to greater than 6, depending on dietary state (fed vs fasted) (35,36). A protocol of repeated 24h periods of fasting and feeding(37) results in prolonged gastric acidity (pH <2) and because concurrent ranitdine reduces lesion severity, it supports the role of acid exposure in pathogenesis. Predominant stimuli to HCl secretion are gastrin, histamine and Ach via vagus nerve (1). Gastrin is released by G cells within gastric mucosa, histamine by mast cells and ECL cells in gastric gland. Histamine binds to type 2 receptors on parietal cell membrane causing an increase in cAMP resulting in phosphorylation of enzymes that activate proton pump. Gastrin and Ach can act via calcium-mediated (Sanchez)
gastric acidity of a horse or foal is very high between periods of eating or nursing. Ulcers in the squamous mucosa result from increased exposure to hydrochloric acid, which can be secondary to prolonged periods of not eating or nursing, intensive exercise, or delayed gastric emptying. (Merck)
Am J Vet Res. 2010 May;71(5):592-6. Expression of cyclooxygenase isoforms in ulcerated tissues of the nonglandular portion of the stomach in horses. Rodrigues NL, Doré M, Doucet MY.
Increased expression of COX-2 in gastric ulcers of the squamous portion of the stomach in horses suggested a role for this enzyme in gastric ulcer healing.
Anatomy Equine gastric ulcers occur in the squamous portion of the stomach in 75-80% of cases. The squamous epithelium, which covers approximately one third of the stomach wall (Fig 21) and the oesophagus, is a relatively simple structure that consists of a tightly bound cornified superficial layer of cells that serves as a protective barrier. The squamous portion of the stomach lining has no absorptive or secretory function, leaving it more vulnerable to peptic injury. The more complex glandular portion of the stomach contains mucus-secreting cells and gastric glands (Fig 221, all of which respond to various stimuli and provide secretions that have a specific function. The gastric glands contain six predominant cell types (Table 3). Physiology The parietal cell secretes H+ via H+/K+ ATPase proton pump (Fig 19) located on its apical membrane. The K+ used by this proton pump and the C1- that combines with the H+ are secreted through the same apical membrane via ion-specific channels. The resultant HCl moves up through the gastric gland and into the gastric lumen, lowering the pH of the gastric contents. Acid and the enzyme pepsin exert peptic influences on digestion. The stomach has a number of protective mechanisms that prevent acid and peptic activity from causing inappropriate mucosal destruction. Glandular mucosal defence mechanisms Two important substances involved in gastric glandular mucosal protection are epidermal growth factors (EGFs) and prostaglandin E2 (PGE2). Epidermal growth factors are found in salivary gland secretions and promote DNA synthesis and proliferation of gastric mucosal cells. They also play a role in prostaglandin synthesis. The PGEz promotes numerous protective functions within the gastric mucosa which include: Suppression of HC1 secretion Mucus secretion Bicarbonate secretion Epithelial restitution mechanisms (maintain tight junctions) Adequate mucosal blood supply. Mucus, secreted by specialised mucous neck cells, is a viscous, hydrophobic glycoproteinaceous gel that adheres to the mucosa and resists acid and pepsin contact. The gel also acts as a lubricant that minimises mechanical damage by gastric contents. Bicarbonate secretion by gastric mucosal cells is triggered by a response to luminal acid concentrations, mechanical irritation, and by release of endogenous prostaglandins. Bicarbonate trapped in the mucous barrier adhering to the stomach wall forms a pH gradient that allows a physiological pH at the mucosal surface and a pH similar to that of stomach acid at the luminal surface. Prostaglandins have an important role in gastric mucosal protection, although their precise mechanism is vague. Prostaglandins inhibit acid secretion, promote mucosal blood flow (vasodilate), increase mucus and bicarbonate secretions, and support mucosal cell repair. Treatment with nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or the administration of prostaglandin antibodies results in ulcer formation in various species, including horses. This effect can be blunted by administering exogenous prostaglandins. Epithelial cell restitution is another important mechanism in the maintenance of gastric mucosal integrity. Epithelial cells act as a protective covering, counter shear forces that induce damage, and provide rapid restoration of damaged protective barriers. In the case of the gastric mucosa, epithelial injury induces a migration of adjacent cells to replace damaged cells. This occurs within minutes without need of new cell proliferation. Shear forces, induced by mixing of ingested material raking against the mucosal wall, cause cell damage that is normally countered by the process of epithelial restoration. An abundant mucosal blood supply is required to provide the mucosa with the oxygen and nutrients necessary to produce the mucus-bicarbonate layer and to support the rapid turnover of epithelial cells. An adequate blood supply is also required to remove acid that has diffused through the mucous layer to the mucosa. Alterations in blood flow (i.e., shock, microvascular thrombosis) are highly correlated with mucosal erosions and gastric ulcers in man. Epidermal growth factors are found in salivary gland secretions and promote DNA synthesis and proliferation of gastric mucosal cells. They also play a role in prostaglandin synthesis and inhibit the parietal gland secretions of hydrochloric acid. Squamous mucosal defence mechanisms Many discussions surrounding equine squamous disease are extrapolated from human oesophageal disease and data from other species. In these species, the initial response of squamous tissues exposed to acid is to thicken. Cell-to-cell junctions are closely adhered (‘tight junctions’), which ensures a weak acid barrier. Buffering also is a component of the squamous tissue defence scheme, but unlike the glandular portion, there is no external mucus-bicarbonate layer. Rather, squamous tissues buffer internally (within the cell) and use leucotrienes for defence. By contrast, glandular tissue relies on prostaglandins for adequate mucosal protection. In summary, the squamous tissues of the stomach have a limited number of defence mechanisms that centre around acid repulsion and intracellular buffering. Once the acid penetrates these defences, it builds up within the cell layers and necrosis (cell death) leads to ulcer formation. Gastric acidity and secretion The horse secretes acid in a continuously variable pattern, such that acid secretion occurs in the absence of ingestion of feed. The mean pH of gastric fluid in horses withheld from feed for several hours has consistently been found to be 2.0 or less. However, periodic fluctuations, in which the pH reaches 6-7 for 5 to 15 min, are commonly seen. Whereas acid output reflects the magnitude of hydrochloric acid secretion, gastric acidity is determined by the pH of gastric secretions and/or contents. The distinction between gastric acid output and pH is relevant, since administration of antisecretory medications may significantly decrease acid output but have minimal effect on gastric fluid pH in horses until the output becomes very small. Few studies on the effect of feeding on gastric physiology have been performed in horses. Serum gastrin increase after feeding was more profound when grain was fed compared with feeding only hay. Horses with free access to hay had greater mean 24 h gastric pH than did horses withheld from feed for 24 h (3.4 * 0.9 vs. 1.9 * 0.5). These latter results support the continuous acid secretion of the equine stomach and the buffering capacity of feed and bicarbonate-rich salivary secretions that are stimulated by feeding. In foals, milk has been demonstrated to have a marked buffering effect.
Extrapolating from other species, causes of glandular ulcer formation would include: Bile reflux into the stomach Hypotensiod shock (resulting in the diminution of mucosal blood flow) Increased sympathetic tone (leading to a decrease in mucosal blood flow) Severe disease states such as uraemia, infections, coagulopathies and conditions that result in the impairment of blood flow Neurological imbalance, resulting in impairment of gastric motility (results in the accumulation of acids) Micro-organisms .(EGUC)
It has been proposed that the disease in a high performance horse is directly linked to unnatural feeding, exercise and management regimes that result in either increased acidity of gastric contents or increased exposure of vulnerable areas of the stomach to acid contents (Murray and Eichorn 1996; Berschneider et al. 1999;Nadeau et al. 2000; Lorenzo-Figueras et al. 2002; Bell et al. 2007). For horses kept in a more natural environment with continuous grass feeding, as some of these were, the aetiology of disease is more puzzling.(Martineau 2009)
We conclude that combinations of bile salts and acid are more injurious to the stratified squamous gastric mucosa of the equine than acid alone. Concentrations of bile salts and acid sufficient to alter the electrolyte transport function of this mucosa can be found in the gastric contents of horses deprived of feed for as little as 14 h.(Berschneider 1999)
In the horse, the junction of the glandular and squamous epithelia is at the margo plicatus, analagous to the gastro-oesophageal junction in man. Therefore, gastric ulceration seen in the equine patient more closely resembles oesophagitis and oesophageal ulceration seen in man (Collier and Stoneham 1997). Severe training regimes may cause diversion of blood flow to muscle, decreasing mucosal blood flow leading to a fall in mucosal resistance.(Collier 1999)
Horses secrete gastric acid continuously, even when they are not eating (Campbell-Thompson and Merritt 1987). Grains contain less buffering material than hay and also contribute an acid load to the system (Roby et al. 1987). There also appears to be an effect of exercise on the stomach that is independent of feeding, as horses fed the same diet before and during training had higher gastrin levels during training (Furr et al. 1994). Whether this is a stress response or some other physiological effect is unknown, but it suggests that exercise itself may be a predisposing factor in the development of gastric ulcers. Stress has been thought to contribute to gastric ulcers or at least to epigastric distress in man and severe stress, associated with illness, may cause ulcers in man and in foals, but only in the glandular portion of the stomach (Furr et al. 1992). (Orsini)
Foal sites of ulceration:
Healthy foals: squamous mucosa, margo plicatus, greater curvature, squamous epithelial desquamation
Sick foals: margo plicatus, lesser curvature and cardia, glandular mucosa, pylorus and duodenum
NOT associated with Helicobacter pylori and not typically associated with Gasterophilus
Foals: hx, cx, gastric reflux, +/- occult blood in faeces, Foals - lesions mainly in glandular epithelium Adults - margo plicatus and squamous epithelium
Risk Factors
Exercise
There appears to be a high prevalence of gastric ulcers in horses performing in most disciplines including racing, endurance, show jumping, dressage and western performance.[29] Although this may be related to exercise, other confounding factors associated with these disciplines such as travel, diet, feeding regime, NSAIDs and stress may be significant. However, Vatistas and co-workers (1999) were able to induce and maintain EGUS in racehorses in fast work without the use of NSAIDs or fasting before exercise.[12] There is also evidence that training for just 8 days is suffcient to induce gastric ulcers.[30] Furthermore, the higher prevalence of gastric ulcers at post mortem in racehorses in training compared to those in retirement adds weight to the hypothesis that exercise is an important risk factor for EGUS.[9] Strenuous exercise is known to stimulate gastrin release which has effects on HCl secretion, gastric emptying and gastric blood flow. It is also thought that exposure of the squamous mucosa to acid is increased as the stomach is compressed by the abdominal viscera and diaphragm during excercise.[31]
Housing and Transport
Housing in stables has been proposed as a risk factor for gastric ulcers, with more lesions being found in confined horses compared to those out at grass.[32] However, when comparing solitary stable confinement with stabling next to a companion, and finally turn out in a paddock, Husted and colleagues (2008) found that the environmental situation had no effect on mucosal acid exposure in the equine stomach.[33] Transport has also been shown to induce squamous mucosal ulceration in horses.[34]
Diet
Feed deprivation encourages gastric ulceration in two ways: (1) it precludes the buffering capacity of protein leading to a reduced gastric pH[35] and (2) it empties the stomach and exposes the squamous mucosa to the more mobile gastric juice.[8] It is unsurprising, therefore, that an alternating feed-fast protocol would produce a consistent model of ulcer induction in the equine squamous mucosa.[36][37] Despite this, feed deprivation is not a prerequisite for gastric ulceration in the horse.[38] Diets that are plentiful in roughage prolong the mastication process and the production of salivary bicarbonate that protects the gastric mucosa. A diet of high grain and low roughage thus predisposes to EGUS.[39] This sort of diet is commonly fed to racehorses but dietary components have also been shown to influence EGUS risk in nonracehorses.[40] Ponies fed a concentrate diet had a greater prevalence of gastric ulcers than ponies fed hay alone.[12] and this may be because grain and pelleted feeds are asssociated with increased serum gastrin.[41] High starch meals are also a risk because they are fermented to volatile fatty acids (VFAs) and lactic acid and are emptied from the stomach relatively slowly.[42][43][44]
Other ailments
Conditions that produce abdominal pain and/or inappetance are likely to reduce food intake and predipose to gastric ulcers.[8] This may be the reason that colic and other gastrointestinal disorders have been associated with EGUS.[45] Alternatively, EGUS may be part of a more general gastrointestinal disease complex.[12] Stress induced by other clinical disorders has been reported to increase the prevalence of EGUS in neonatal foals[46] and a similar mechanism may exist for adult animals.[12]
NSAIDs
As in other species, nonsteroidal anti-inflammatory drugs (NSAIDs) have been shown to cause gastric ulcers in horses. Typicaly this is associated with high doses or frequent administration of phenylbutazone or flunixin meglumine. However, although there is evidence to the contrary,[47]therapeutic doses of NSAIDs may be sufficient to induce EGUS. Other studies have suggested that suxibuzone causes significantly less ulcerogenic effects than phenylbutazone when administered orally[48]and that combination treatment with phenylbutazone and flunixin meglumine may be more risky than phenylbutazone alone.[49] The ulcers produced by NSAIDs are unusual in that they have a predilection for the glandular mucosa[50][51][52], they may look different endoscopically from ulcers that occur naturally,[53] and they appear to heal spontaneously.[54][55] Despite the well-established link bewteen NSAIDs and ulcers, NSAIDs are rarely responsible for the lesions in horses in race training.[56][57][38]
Temperament
A nervous disposition has been linked with gastric ulcers[58]but the same association was not seen in another study.[59] The physiological and psychological stresses of training, housing, boredom, travel, mixing, hospitalisation and entering new environments[12] may increase the risk of developing EGUS. In foals hypoxia may also be a risk factor.
Clinical syndrome
The clinical signs associated with gastric ulcers are often very non-sepcific, difficult to document and at times only subjective.[60] In addition, there appears to be a poor correlation between the severity of endoscopic lesions and the clinical presentation.[20] The significance of gastric ulceration in horses thus remains questionable. However, there have been instances where ulcer treatment has preceded an improvement in clinical status and/or racing perfomance, suggesting that in some horses, ulcers are a considerable burden.[60] Cases gastric ulceration are often asymptomatic, but signs that have been attributed to these lesions in mature horses include:
- Poor appetite (particularly decreased consumption of concentrates)[6]
- Poor condition
- Rough hair coat
- Weight loss
- Excessive recumbency[2]
- Mild to severe colic
- Changes in attitude (dullness or depression)[60]
- Poor racing performance and reluctance to train
Clinical signs in foals vary depending on age and severity:
- Neonatal foals: many ulcers are silent, some foals only exhibit signs when ulceration has become severe. Glandular ulcers are considered the most significant[6]
- Poor appetite
- Diarrhoea
- Intermittent colic
- Frequent dorsal recumbency
- Sucklings and weanlings:[6]
- Diarrhoea
- Poor appetite (off suck or partially off suck)
- Poor growth, failure to thrive
- Poor body condition
- Rough hair coat
- Potbelly appearance
- Bruxism (almost pathognomonic)
- Colic after feeding or tubing
- Chewing straw
- Dorsal recumbency
- Signs of gastroduodenal ulcer disease (GDUD):[6]
- Bruxism
- Colic
- Gastrooesophageal reflux after suckling
- Ptyalism (secondary to gastric outflow obstruction and gastroesophageal reflux)[7]
- Diarrhoea
In foals with outflow obstruction distal to the common bile duct, marked reflux may be seen even with limited nursing.[6] GDUD is the primary differential for ptyalism in foals, other possible diagnoses include oesophageal obstruction and Candida infection.[7]
Diagnosis
A presumptive diagnosis can be based on clinical signs and response to therapy,[6] however, a definitive diagnosis requires visualisation of the stomach. This can be achieved in the live horse using endsocopy or, alternatively, at post-mortem.[39]
EGUS was recently discussed at the 2010 Annual meeting between the Equine Insurers Forum (EIF) and the British Equine Veterinary Association (BEVA). The EIF maintained that in order to support claims for the long term costs associated with treatment of EGUS, there would be a requirement for veterinary surgeons to make a definitive diagnosis prior to prescribing omeprazole.
Endoscopy
Oesophagogastroscopy or duodenoscopy can be performed under mild sedation (e.g. 0.6-0.8mg/kg xylazine[60]) in the standing horse. Of these, duodenoscopy is the more specific but more technically demanding method.[6] Endoscopic examination requires preparatory starving of the patient for 6-8hours,[60] eliciting a certain degree of stress. As such, it is preferable not to carry out this technique in foals. In adult horses, a minimum endoscope length of two metres is essential to visualize the gastric body and fundus.[6] A 2.8-3.0 metre endoscope is needed to observe the gastric antrum, pylorus and proximal dudoenum.[6] In either case, fibreoptic or videoendoscopic equipment can be used.[2]
Based on a consensus, the Equine Gastric Ulcer Council (EGUC) published an EGUS Lesion Scoring System which they claimed to be simple and applicable to both regions of the equine gastric mucosa.[2] This last point has been debated, since most of the acquired data on gastric lesions refers only to the squamous mucosa.[1] At the time of writing however, the EGUC system appears to be the most well established and useful in practice:
| Lesion Grade | Description |
| Grade 0 | Intact epithelium with no appearance of hyperaemia (reddening) or hyperkeratosis (yellowing of the squamous mucosa) |
| Grade 1 | Intact mucosa with areas of reddening or hyperkeratosis (squamous) |
| Grade 2 | Small single of multifocal lesions |
| Grade 3 | Large single or multifocal lesions or extensive superficial lesions |
| Grade 4 | Extensive lesions with areas of deep ulceration |
Diffuse inflammation may be the only lesion observed in foals with early GDUD.[6] In contrast to other scoring systems,[65] the EGUC approach does not include bleeding when assigning lesion grades. The justification is that the 'snapshot' provided by endoscopy may by chance identify bleeding of superficial erosions whilst missing the intermittent haemorrhage of more severe lesions. [2] Endoscopy may assist in understanding the severity of the disease and assessing the therapeutic response, but it is not without disadvantages. Ulcer severity may be underestimated, particularly in the squamous region and glandular ulcers may be missed altogether.[66] Lesions that appear grossly similar may have different grades on histopathology.[39] This is important as varying lesions may have different causes, requiring a range of treatment approaches.
Radiography
In older foals with GDUD, detection of gastric outflow obstruction via abdominal radiography is essential to treatment and prognosis.[6] Liquid barium will demonstrate very delayed or no outflow depending on the degree of obstruction. Without contrast medium, a large, gas filled stomach will be obvious.[6]. The need to perform contrast radiography must be weighed against the stress it would place upon the foal.
Biopsy
A transendoscopic gastric biopsy technique was recently validated for obtaining samples from the gastric glandular mucosa in the live horse.[67]Unfortunately this technique failed to produce samples of squamous mucosa that would be suitable for histopathological analysis.
Laboratory tests
Currently, useful and reliable markers for EGUS are lacking.[2] The SUCCEED® Equine Fecal Blood Test™ uses specific equine monoclonal antibodies to albumin and haemoglobin to detect occult blood in faeces.[69][70]The test has a positive predictive value of 77% and a negative predictive value of 72% and thus cannot be relied upon alone to diagnose EGUS.[39] False positive results may arise from rectal trauma (e.g. recent biopsy or rectal examination) or protein losing enteropathy.[39] Other tests that require further analysis for sensitivity and specificity[6] include:
- Urine[71] and blood[72] sucrose absorption as an assay of gastric mucosal permeability
- Serum alpha1-antitrypsin may be released from damaged gastric tissue[39] and has been detected more frequently in foals with gastric ulceration[73]
Pathology
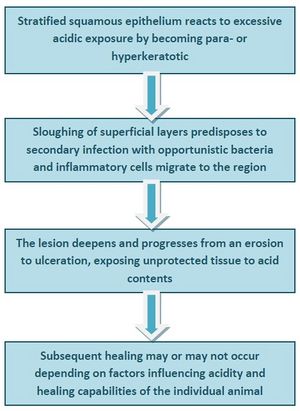
Martineau and co-workers (2009) demonstrated that in a mixed population of horses, a wide range of lesions associated with EGUS could be found at post-mortem.[5] These included hyperkeratosis, punctate scars, diffuse erosions or ulcerations and margo injuria in the squamous region and hyperaemia, focal erosions and ulcerations in the glandular region. A novel finding was glandular metaplasia which may be evidence of a protective mechanism developing in response to acid exposure.[5] The authors then devised a pathological scoring system - the Equine Gastritis Grading (EGG) system - which uses 5 samples of gastric mucosa taken from specific regions of the equine stomach. For each of these, the inflammatory infiltrate is graded by type, density and location, reactive changes are classified in both squamous and glandular samples and the presence or absence of infectious agents and lymphoid follicles is noted.[68] From their findings, a pathogenesis for the development of lesions in the squamous region was proposed:
Treatment
Histamine 2 receptor antagonists
Parietal cells secrete HCl upon stimulation of histamine, acetylcholine or gastrin receptors.[2] Competitive H2 receptor antagonists have successfully elevated gastric pH and treated gastric ulcers in mature horses and foals.(44,85,97 in Sachez) There appears to be a great variability among horses in their dose requirements for H2 antagonists which may be explained by individual bioavilability for these compounds.[2] Currently recommended doses proposed to be effective in the majority of horses[6] are:
- Cimetidine 20-30mg/kg PO every 8 hours or 6.6mg/kg IV every 6 hours
- Ranitidine 6.6mg/kg PO every 8 hours or 1.5-2mg/kg IV every 6 hours
- Famotidine 10-15mg/kg PO every 24 hours
Proton-pump inhibitors (PPIs)
PPIs irreversibly bind to the H+K+-ATPase proton pump of the parietal cell and block the secretion of hydrogen ions. These agents are more effective than H2 antagonsists as their action is receptor-independent,[2] blocking the final pathway of acid secretion and they have a prolonged effect allowing for once-daily dosing.((Brown and Rees 1994). Papich 1993, Sanchez) Omeprazole (Gastroguard™), a subsituted benzimidazole, is currently the only PPI licensed for use in horses. At a dose rate of 4mg/kg per day omeprazole has proven effective in reducing the severity of gastric ulcers in Thoroughbred horses in active race training[74] and no adverse effects have been observed. The paste formulation is easy to administer and generally well accepted by horses. Omeprazole has demonstrated efficacy in the resolution ofboth naturally-occurring and NSAID-induced gastric ulcers in horses.(103.104 in Sanchez) A single dose has also produced an increase in gastric pH in clinically ill neonatal foals[75] and has contributed to ulcer healing in neonates.[76] A potential concern is that altering gastric pH may encourage bacterial overgrowth. Thus further work is needed to evaluate the long-term safety of omeprazole in horses and particularly, foals.[74]
Antacids
The use of antacids to treat EGUS in the horse has not been critically evaluated[6] and some believe they are contraindicated due to potential rebound effects. Furthermore, the requirement for frequent dosing of large volumes of these products (owing to their poor efficacy) makes them an unattractive, stressful and impractical alternative to omeprazole.[60]
Mucosal protectants
Sucralfate is a complex salt of sucrose and aluminium hydroxide. It is thought to promote ulcer healing via several mechanisms: adherence to ulcerated mucosa, stimulation of mucus secretion, pepsin inibition, increasing prostgalandin E synthesis and enhancing the local production of epidermal growth factor.[6] It has been used effectively to treat and prevent stress-induced ulcers in man and has been recommended at 10-20mg/kg three times daily for the treatment of glandular ulcers in horses.[77] However, the effect of sucralfate on equine squamous gastric ulcers remains inconclusive[2] and the product may be ineffective in the alkaline conditions created by acid suppression agents.(123-125 in Sanchez)
Prostaglandin analogues
Synthetic prostaglandin E1 analogues are believed to inihibit gastric acid secretion and enhance mucosal cytoprotection.[78] Misoprostol has been an effective agent in the treatment of human gastric and duodenal ulcers and at 5µg/kg has been shown to increase gastric pH in horses.[79] Although contraindicated in pregnant mares, Misoprostol may be beneficial for mucosal recovery in the face of flunixin treatment.[80]
Gastric prokinetics
In cases of gastrooesophageal reflux, duodenal disease and delayed gastric emptying without a serious physical obstruction to gastric outflow, gastric prokinetics might be considered.[6] Such compounds include bethanechol, metaclopramide, erythromycin and cisapride which have been shown to hasten gastric empyting in adult horses.[2] To date only the parasympathomimetic agent bethanechol has been used as an adjunct for EGUS and cholinergic side effects are possible. Cisapride has been withdrawn from the US and UK markets over concern about its potential to cause adverse cardiac effects in man.[6]
Treatment problems
The prevalence of gastric ulcers in horses remains high regardless of the common use of antiulcer treatments. This has been attributed to the expense of recommended products encouraging subtherapeutic and curtailed dosing schedules(Orsini et al 2003 in Nadeau 2009). Omeprazole and ranitidine must be administered for at least 28 days for adequate ulcer healing.[39] In the USA, compounded omeprazole from bulk powders are used as a cheaper substitute for the FDA approved products. However, these formulations lack efficacy and are not regulated (Nieto et al. 2002; Merritt et al. 2003; Orsini et al. 2003).[39] A considerable challenge lies in the management of abdominal pain associated with EGUS, since the commonly used NSAIDs for pain control may worsen and even induce further ulcerative lesions.[81] Another challenge is the horse in which oral medication is prohibited. However, Andrews and colleagues (2006) have demonstrated the efficacy of an omeprazole powder, adminstered IV in sterile water, which signifcantly increases the pH of equine gastric contents and may be useful in problem horses.[82] An ongoing point of debate is the use of antiulcer medication in competition horses. In 2000, the Bureau of the The Fèdèration Equestre Internationale (FEI) permitted the use of cimetidine, ranitidine and omeprazole to prevent and treat gastric ulcers. This decision was based on evidence that the compounds were not performance enhancing and that EGUS was such a widespread concern. However, these drugs are still listed under prohibited substances in the 2009 Appendices of the American Endurance Ride Conference (AERC) Rules and Regulations. The argument is that a horse requiring such treatment is not suffciently well to compete and should be withdrawn form competition if it needs preventative medication. A related concern is that the AERC permits the use of hyperosmolar oral electrolyte pastes which may cause gastric ulcers.(Holbrook et al. 2005) Without the protection afforded by antiulcer agents, these horses may be at considerable risk for EGUS.[39]
Prognosis
Improvement in most clinical signs should be noted within 1-3 weeks of commencing treatment. Colic or diarrhoea should resolve within 48 hours.[6] Complications related to gastric ulcers are most frequent and severe in foals and include perforation, delayed gastric emptying, gastroesophageal reflux and oesophagitis, and megaoesophagus secondary to chronic gastroesophageal reflux. Sudden gastric perforation without prior signs occurs sporadically in foals.[7]Ulcers in the proximal duodenum or at the pylorus can cause fibrosis and stricture. The latter complication is seen in both foals and adult horses.[7] In mature animals, the most common complication is the recurrence of EGUS after treatment has ceased. This is typically because the inciting managemental causes have not been altered.
Prevention
Management
- Diet: ideally turnout to good quality grass.(Murray 1994) Stabled horses should have continuous access to hay and should be offered this before calorifc needs are met by concentrates.[60] Alfalfa, or another high calcium or high protein forage may be preventative by increasing gastric pH.(Nadeau et al. 2000; Lybbert et al. 2007; Ralston 2007) Concentrates should be fed at no more than 0.5kg per 100kg body weight and not more frequently than every 6 hours..[82] Horses prone to, or at risk of, EGUS should be fed the minimum amount of concentrates necessary.[39]
- Stress: minimise handling wherever possible, provide company and toys for stabled horses, encourage good feeding habits of foals.
Most of these suggestions would be difficult if not impossible to achieve for horses in race training, thus prophylactic medication should be considered.[60]
Prophylaxis
Omeprazole paste at a lower dose (1-2mg/kg) daily for 3-4 weeks.(100, 107-109 in Sanchez)
- Prevented ulcers in horses maintained under ulcerogenic conditions (White et al. 2003; McClure et al. 2005a,b,c;White et al. 2007).
- Treating ulcers in asymptomatic performance horses may lead to improved performance.[60]
- Prophylaxis in foals controversial as gastric acidity may be protective against bacterial translocation.[6]
- May benefit foals receiving substantial doses of NSAIDs for orthopaedic pain.[6]
References
- ↑ 1.0 1.1 Merritt, A M (2009) Appeal for proper usage of the term ʻEGUSʼ: Equine gastric ulcer syndrome. Equine Vet J, 41(7):616.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 The Equine Gastric Ulcer Council (1999) Tutorial Article: Recommendations for the diagnosis and treatment of equine gastric ulcer syndrome (EGUS). Equine Vet Educ, 11(5):262-272.
- ↑ Andrews, F.M, Bernard, W.V, Byars, T.D et al. (1999) Recommendations for the diagnosis and treatment of equine gastric ulcer syndrome (EGUS). Equine Vet Educ, 1:122-134. In: Sanchez, L.C (2010) 'Diseases Of The Stomach' in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 15.
- ↑ 4.0 4.1 Bell, R.J, Mogg, T, Kingston, J.K (2007) Equine gastric ulcer syndrome in adult horses: a review. N Z Vet J, 55(1):1-12).
- ↑ 5.0 5.1 5.2 Martineau, H, Thompson, H, Taylor, D (2009) Pathology of gastritis and gastric ulceration in the horse. Part 1: Range of lesions present in 21 mature individuals. Equine Vet J, 41(7):638-644. Cite error: Invalid
<ref>tag; name "Martineau" defined multiple times with different content Cite error: Invalid<ref>tag; name "Martineau" defined multiple times with different content - ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 6.15 6.16 6.17 6.18 6.19 6.20 6.21 6.22 6.23 Sanchez, L.C (2010) 'Diseases Of The Stomach' in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 15.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 Merck & Co (2008) The Merck Veterinary Manual (Eighth Edition), Merial
- ↑ 8.0 8.1 8.2 8.3 8.4 Sandin, A, Skidell, J, Haggstrom, J, Nilsson, G (2000) Postmortem findings of gastric ulcers in Swedish horses older than age one year: a retrospective study of 3715 horses (1924–1996). Equine Vet J, 32(1):36-42.
- ↑ 9.0 9.1 9.2 9.3 Hammond, C.J, Mason, D.K, Watkins, K.L (1986) Gastric ulceration in mature Thoroughbred horses. Equine Vet J, 18(4):284-287.
- ↑ Vatistas, N.J, Snyder, J.R, Carlson, G, et al (1994) Epidemiological study of gastric ulceration in the thoroughbred racehorse:202 horses 1992-1993. Proc Am Assoc Equine Pract, 40:125-126
- ↑ Murray, M.J, Schusser, G.F, Pipers, F.S, Gross, S.J (1996) Factors associated with gastric lesions in thoroughbred racehorses. Equine Vet J, 28:368-374.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 Vatistas, N.J, Sifferman, R.L, Holste, J, Cox, J.L, Pinalto, G, Schultz, K.T (1999) Induction and maintenance of gastric ulceration in horses in simulated race training. Equine Vet J Suppl, 29:40-44
- ↑ Vatistas, N.J, Snyder, J.R, Carlson, G, Johnson, B, Arthruy, R.M, Thurmond, M, Zhou, H, Lloyd, K.L.K (1999) Cross-sectional study of gastric ulcers of the squamous mucosa in Thoroughbred racehorses. Equine Vet J, Suppl 29:34-39.
- ↑ McClure, S.R, Glickman, L.T, Glickman, N.W (1999) Prevalence of gastric ulcers in show horses. J Am Vet Med Assoc, 215:1130-1133.
- ↑ MacAllister, C.G, Sangiah, S, Mauromoustakos, A (1992) Effect of a histamine H, type receptor antagonist (WY 45, 727) on the healing of gastric ulcers in ponies. J Vet Int Med, 6:271-275.
- ↑ Nieto, J.E, Snyder, J.R, Beldomenico, P et al. (2004) Prevalence of gastric ulcers in endurance horses: a preliminary report. Vet J, 167:33-37.
- ↑ Bertone, J (2000) Prevalence of gastric ulcers in elite, heavy use western performance horses. Proc Am Assoc Equine Pract, 46:256-259.
- ↑ LeJeune, S.S, Nieto, J.E, Dechant, J.E, Snyder, J.R (2009) Prevalence of gastric ulcers in Thoroughbred broodmares in pasture: a preliminary report. Vet J, 181(3):251-5.
- ↑ Hartmann, A.M, Frankeny, R.L (2003) A preliminary investigation into the association between competition and gastric ulcer formation in non-racing performance horses. J Equine Vet Sci, 23:560-561. In:Sanchez, L.C (2010) 'Diseases Of The Stomach' in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 15.
- ↑ 20.0 20.1 Murray, M.J, Grodinsky, C, Anderson, C.W, Radue, P.F, Schmidt, G.R (1989) Gastric ulcers in horses: a comparison of endoscopic findings in horses with and without clinical signs. Equine Vet J Suppl, 7:68-72.
- ↑ Wilson, J.H (1986) Gastric and duodenal ulcers in foals: a retrospective study. Proc Equine Colic Res Symp 2nd:126-128.
- ↑ Murray, M.J, Grodinsky, C, Cowles, R.R, et al.(1990) Endoscopic evaluation of changes in gastric lesions of Thoroughbred foals. J Am Vet Med Assoc, 196:1623-1627.
- ↑ Murray, M.J (1989) Endoscopic appearance of gastric lesions in foals: 94 cases (1987-1988). J Am Vet Med Assoc, 195:1135-1141.
- ↑ Orsini, J.A, Pipers, F.S (1997) Endoscopic evaluation of the relationship between training, racing, and gastric ulcers. Vet Surg, 26:424. In: Orsini, J (2000) Tutorial Article Gastric ulceration in the mature horse: a review. Equine Vet Educ, 12(1):24-27.
- ↑ Murray, M.J (1994) Gastric ulcers in adult horses. Comp Cont Educ Pract Vet, 16:792-794. In:Orsini, J (2000) Tutorial Article Gastric ulceration in the mature horse: a review. Equine Vet Educ, 12(1):24-27.
- ↑ Murray, M. (1992) Gastric ulceration in horses: 91 cases (1987-1990). J Am Vet Med Assoc, 201:117-120. In: Martineau, H, Thompson, H, Taylor, D (2009) Pathology of gastritis and gastric ulceration in the horse. Part 1: Range of lesions present in 21 mature individuals. Equine Vet J, 41(7):638-644.
- ↑ Luthersson, N, Nielsen, K.H, Harris, P, Parkin, T.D (2009) The prevalence and anatomical distribution of equine gastric ulcer syndrome (EGUS) in 201 horses in Denmark. Equine Vet J, 41(7):619-24.
- ↑ Murray, M.J (1994) Characteristics of gastric ulcer pathophysiology. Proc Am Coll Vet Intern Med, 12:610-612. In: Sandin, A, Skidell, J, Haggstrom, J, Nilsson, G (2000) Postmortem findings of gastric ulcers in Swedish horses older than age one year: a retrospective study of 3715 horses (1924–1996). Equine Vet J, 32(1):36-42.
- ↑ Hartmann, A.M, Frankeny, R.L (2003) A preliminary investigation into the association between competition and gastric ulcer formation in non-racing performance horses. J Equine Vet Sci, 23:560-561. In: Nadeau, J.A, Andrews, F.M (2009) Science: Overviews Equine gastric ulcer syndrome: The continuing conundrum. Equine Vet J, 41(7):611-615.
- ↑ White, G, McClure, S.R, Siifferman, R, Holste, J.E, Fleishman, C, Murray, M.J, Cramer, L.G (2007) Effects of short-term light to heavy exercise on gastric ulcer development in horses and efficacy of omeprazole paste in preventing gastric ulceration. J Am Vet Med Assoc, 230(11):1680-2.
- ↑ Lorenzo-Figueras, M, Merritt, A.M (2002) Effects of exercise on gastric volume and pH in the proximal portion of the stomach of horses. Am J Vet Res, 63:1481-1487.
- ↑ Murray, M.J, Eichorn, E.S (1996) Effects of intermittent feed deprivation, intermittent feed deprivation with ranitidine administration, and stall confinement with ad libitum access to hay on gastric ulceration in horses. Am J Vet Res, 57:1599-1603.
- ↑ Husted, L, Sanchex, L.C, Olsen, S.N, Baptiste, K.E, Merritt, A.M (2008) Effect of paddock vs. stall housing on 24 hour gastric pH within the proximal and ventral equine stomach. Equine Vet J, 40(4):337-41.
- ↑ McClure, S.R, Carithers, D.S, Gross, S.J, Murray, M.J (2005) Gastric ulcer development in horses in a simulated show or training environment. J Am Vet Med Assoc, 227:775-777.
- ↑ Murray, M.J, Schusser, G.F (1993) Measurement of 24-h gastric pH using an indwelling pH electrode in horses unfed, fed and treated with ranitidine. Equine Vet J, 25:417-421. In: Sandin, A, Skidell, J, Haggstrom, J, Nilsson, G (2000) Postmortem findings of gastric ulcers in Swedish horses older than age one year: a retrospective study of 3715 horses (1924–1996). Equine Vet J, 32(1):36-42.
- ↑ Murray, M.J, Schusser, G.F (1993) Measurement of 24-h gastric pH using an indwelling pH electrode in horses unfed, fed and treated with ranitidine. Equine Vet J, 25:417-421. In: Sanchez, L.C (2010) 'Diseases Of The Stomach' in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 15.
- ↑ Murray, M.J (1994) Equine model of inducing ulceration in alimentary squamous epithelial mucosa. Dig Dis Sci, 39:2530-2535. In: Sanchez, L.C (2010) 'Diseases Of The Stomach' in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 15.
- ↑ 38.0 38.1 Vatistas, N.J (1998) Gastric Ulceration in the Racing Thoroughbred. PhD Thesis. In: Vatistas, N.J, Sifferman, R.L, Holste, J, Cox, J.L, Pinalto, G, Schultz, K.T (1999) Induction and maintenance of gastric ulceration in horses in simulated race training. Equine Vet J Suppl, 29:40-44
- ↑ 39.0 39.1 39.2 39.3 39.4 39.5 39.6 39.7 39.8 39.9 In: Nadeau, J.A, Andrews, F.M (2009) Science: Overviews Equine gastric ulcer syndrome: The continuing conundrum. Equine Vet J, 41(7):611-615. Cite error: Invalid
<ref>tag; name "Nadeau" defined multiple times with different content Cite error: Invalid<ref>tag; name "Nadeau" defined multiple times with different content Cite error: Invalid<ref>tag; name "Nadeau" defined multiple times with different content Cite error: Invalid<ref>tag; name "Nadeau" defined multiple times with different content Cite error: Invalid<ref>tag; name "Nadeau" defined multiple times with different content Cite error: Invalid<ref>tag; name "Nadeau" defined multiple times with different content Cite error: Invalid<ref>tag; name "Nadeau" defined multiple times with different content Cite error: Invalid<ref>tag; name "Nadeau" defined multiple times with different content Cite error: Invalid<ref>tag; name "Nadeau" defined multiple times with different content - ↑ Luthersson, N, Nielson, K.H, Harris, P, Parkin, T.D (2009) Risk factors associated with equine gastric ulceration syndrome (EGUS) in 201 horses in Denmark. Equine Vet J, 41(7):625-30.
- ↑ Smyth, G.B, Young, D.W, Hammond, L.S (1988) Effects of diet and feeding on post-prandial serum gastrin and insulin concentrations in adult horses. Equine Vet J Suppl 7:56-59.
- ↑ Mètayer, N, Lhôte, M, Bahr, A, Cohen, N.D, Kim, I, Rousell, A.J, Julliand, V (2004) Meal size and starch content affect gastric emptying in horses. Equine Vet J, 36:434-440. In: Nadeau, J.A, Andrews, F.M (2009) Science: Overviews Equine gastric ulcer syndrome: The continuing conundrum. Equine Vet J, 41(7):611-615.
- ↑ Taharaguchi, S, Okai, K, Orita, Y, Kuwano, M, Ueno, T, Taniyama, H (2004) Relation between amounts of concentrated feed given mares and gastric ulcers in foals. J Japan Vet Med Ass, 57:366-370. In: Nadeau, J.A, Andrews, F.M (2009) Science: Overviews Equine gastric ulcer syndrome: The continuing conundrum. Equine Vet J, 41(7):611-615.
- ↑ Boswinkel, A.M, Ellis, A.D, Sloet van Oldruitenborgh-Oosterbaan, M.M (2007) The influence of low versus high fibre haylage diets in combination with training or pasture rest on equine gastric ulceration syndrome (EGUS). Pferdeheilkunde, 23:123-130. In: Nadeau, J.A, Andrews, F.M (2009) Science: Overviews Equine gastric ulcer syndrome: The continuing conundrum. Equine Vet J, 41(7):611-615.
- ↑ Furr, M.O, Murray, M.J (1989) Treatment of gastric ulcers in horses with histamine type 2 receptor antagonists. Equine Vet J Suppl, 7:77-79.
- ↑ Furr, M.O, Murray, M.J, Ferguson, D.C (1992) The effects of stress on gastric ulceration, T3, T4, reverse T3 and cortisol in neonatal foals. Equine Vet J, 24:37-40.
- ↑ Andrews, F.M, Reinemeyer, C.R, Longhofer, S.L (2009) Effects of top-dress formulations of suxibuzone and phenylbutazone on development of gastric ulcers in horses. Vet Ther, 10(3):113-20.
- ↑ Monreal, L, Sabatè, D, Segura, D, Mayós, I, Homedes, J (2004) Lower gastric ulcerogenic effect of suxibuzone compared to phenylbutazone when administered orally to horses. Res Vet Sci, 76:145-149. In: Nadeau, J.A, Andrews, F.M (2009) Science: Overviews Equine gastric ulcer syndrome: The continuing conundrum. Equine Vet J, 41(7):611-615.
- ↑ Reed, S.K, Messer, N.T, Tessman, R.K, Keegan, K.G (2006) Effects of phenylbutazone alone or in combination with flunixin meglumine on blood protein concentrations in horses. Am J Vet Res, 67:398-402. In: Nadeau, J.A, Andrews, F.M (2009) Science: Overviews Equine gastric ulcer syndrome: The continuing conundrum. Equine Vet J, 41(7):611-615.
- ↑ MacAllister, C.G, Morgan, S.J, Borne, A.T, Pollet, R.A, (1993) Comparison of adverse effects of phenylbutazone, flunixin meglumine, and ketoprofen in horses. J Am Vet Med Ass, 202:71-77. In: Jonsson, H, Egenvall, A (2006) Prevalence of gastric ulceration in Swedish Standardbreds in race training. Equine Vet J, 38(3):209-213.
- ↑ Furr, M.O, Murray, M.J (1989) Treatment of gastric ulcers in horses with histamine type 2 receptor antagonists. Equine Vet J Suppl, 7:77-79. In: Vatistas, N.J, Sifferman, R.L, Holste, J, Cox, J.L, Pinalto, G, Schultz, K.T (1999) Induction and maintenance of gastric ulceration in horses in simulated race training. Equine Vet J Suppl, 29:40-44
- ↑ Kumaran, D, Bhuvanakumar, C.K (1994) Gastro duodenal ulceration in foals - a discussion. Cenfaur Mylapore, 10:83-86. In: Vatistas, N.J, Sifferman, R.L, Holste, J, Cox, J.L, Pinalto, G, Schultz, K.T (1999) Induction and maintenance of gastric ulceration in horses in simulated race training. Equine Vet J Suppl, 29:40-44
- ↑ Jonsson, H, Egenvall, A (2006) Prevalence of gastric ulceration in Swedish Standardbreds in race training. Equine Vet J, 38(3):209-213.
- ↑ Jones, W.E (1983) Gastrointestinal ulcers [foal]. Equine Vet Data, 4:305-308. In: Vatistas, N.J, Sifferman, R.L, Holste, J, Cox, J.L, Pinalto, G, Schultz, K.T (1999) Induction and maintenance of gastric ulceration in horses in simulated race training. Equine Vet J Suppl, 29:40-44
- ↑ MacAllister, C.G, Sangiah, S (1993) Effect of ranitidine (in healing of experimentally induced gastric ulcers in ponies. Am J Vet Res, 54:1103-1107. In: Vatistas, N.J, Sifferman, R.L, Holste, J, Cox, J.L, Pinalto, G, Schultz, K.T (1999) Induction and maintenance of gastric ulceration in horses in simulated race training. Equine Vet J Suppl, 29:40-44
- ↑ Vatistas N.J, Snyder, J.R, Carlson, G.P, Johnson, B, Arther, R.M, Thurmiind, M, Lloyd, K.C.K (1994) Epidemiology study of gastric ulcerarion in the Thoroughbred race horse: 202 horses. Proc Am Ass Equine Pract, 39:125-126. In: Vatistas, N.J, Sifferman, R.L, Holste, J, Cox, J.L, Pinalto, G, Schultz, K.T (1999) Induction and maintenance of gastric ulceration in horses in simulated race training. Equine Vet J Suppl, 29:40-44
- ↑ Murray, M.J, Schusser, G.F, Pipers, F.S, Gro:ss, S.J (1996) Factors associated with gastric lesions in Thoroughbred racehorses. Equine Vet J, 28:368-374. In: Vatistas, N.J, Sifferman, R.L, Holste, J, Cox, J.L, Pinalto, G, Schultz, K.T (1999) Induction and maintenance of gastric ulceration in horses in simulated race training. Equine Vet J Suppl, 29:40-44
- ↑ McClure, S.R, Glickman, L.T, Glickman, N.W (1999) Prevalence of gastric ulcers in show horses. J Am Vet Med Ass 215:1130-1133. In: In: Jonsson, H, Egenvall, A (2006) Prevalence of gastric ulceration in Swedish Standardbreds in race training. Equine Vet J, 38(3):209-213.
- ↑ Vatistas, N.J, Snyder, J.R, Carlson, G, Johnson, B, Arthur, R.M, Thurmond, M, Zhou, H, Lloyd, L.K (1999) Cross-sectional study of gastric ulcers of the squamous mucosa in Thoroughbred racehorses. Equine Vet J Suppl, 29:34-39. In: Jonsson, H, Egenvall, A (2006) Prevalence of gastric ulceration in Swedish Standardbreds in race training. Equine Vet J, 38(3):209-213.
- ↑ 60.0 60.1 60.2 60.3 60.4 60.5 60.6 60.7 60.8 Orsini, J (2000) Tutorial Article Gastric ulceration in the mature horse: a review. Equine Vet Educ, 12(1):24-27.
- ↑ Videla, R, Andrews, F.M (2009) New perspectives in equine gastric ulcer syndrome. Vet Clin North Am Equine Pract, 25(2):283-301.
- ↑ Dukti, S.A, Perkins, S, Murphy, J, Barr, B, Boston, R, Southwood, L.L, Bernard, W (2006) Prevalence of gastric squamous ulceration in horses with abdominal pain. Equine Vet J, 38:347-349.
- ↑ Franklin, S.H, Brazil, T.J, Allen, K.J (2008) Poor performance associated with equine gastric ulceration syndrome in four Thoroughbred racehorses. Equine Vet Educ, 20:119-124. In: Nadeau, J.A, Andrews, F.M (2009) Science: Overviews Equine gastric ulcer syndrome: The continuing conundrum. Equine Vet J, 41(7):611-615.
- ↑ Nieto, J.E, Snyder, J.R, Vatistas, N.J, Jones, J.H (2009) Effect of gastric ulceration on physiologic responses to exercise in horses. Am J Vet Res, 70(6):787-95.
- ↑ MacAllister, C.G, Andrews F.M, Deegan E, Ruoff, W, Olovson, S.G (1997) A scoring system for gastric ulcers in horses. Equine Vet J, 29:430-433.
- ↑ Andrews, F.M, Reinmeyers, C.R, McCracken, M.D, Blackford, J.T, Nadeau, J.A, Saabye, L, Sotell, M, Saxton, A (2002) Comparison of endoscopic, necropsy and histology scoring of equine gastric ulcers. Equine Vet J,34(5):475-478.
- ↑ Rodrigues, N.L, Dore, M, Doucet, M.Y (2009) Validation of a transendoscopic glandular and nonglandular gastric biopsy technique in horses. Equine Vet J, 41(7):631-5.
- ↑ 68.0 68.1 Martineau, H, Thompson, H, Taylor, D (2009) Pathology of gastritis and gastric ulceration in the horse. Part 2: a scoring system. Equine Vet J,41(7):646-51.
- ↑ Carter, S, Pellegrini, F.A (2006) The use of novel antibody tools to detect the presence of blood in equine feces. Company Bulletin Freedom Health LLC 1-3. In: Nadeau, J.A, Andrews, F.M (2009) Science: Overviews Equine gastric ulcer syndrome: The continuing conundrum. Equine Vet J, 41(7):611-615.
- ↑ Pellegrini, F.L, Carter, S.D (2007) An equine necroscopic study to determine the sensitivity and specificity of a dual antibody test. Company Bulletin Freedom Health LLC 1-2. In: Nadeau, J.A, Andrews, F.M (2009) Science: Overviews Equine gastric ulcer syndrome: The continuing conundrum. Equine Vet J, 41(7):611-615.
- ↑ O'Connor, M.S, Steiner, J.M, Roussel, A.J, et al. (2004) Evaluation of urine sucrose concentration for detection of gastric ucleration in horses. Am J Vet Res, 65:31-39. In: Sanchez, L.C (2010) 'Diseases Of The Stomach' in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 15.
- ↑ Hewetson, M, Cohen, N.D, Love, S, et al. (2006) Sucrose concentration in bood: a new method for assessment of gastric permeability in horses with gastric ulceration. J Vet Intern Med, 20:388-394. In: Sanchez, L.C (2010) 'Diseases Of The Stomach' in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 15.
- ↑ Taharaguchi, S, Nagano, A, Okai, K, et al. (2007) Detection of an isoform of alpha(1)-antitrypsin in serum samples from foals with gastric ulcers. Vet Rec, 161:338-342. In: Sanchez, L.C (2010) 'Diseases Of The Stomach' in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 15.
- ↑ 74.0 74.1 Vatistas, N.J, Snyder, J.R, Nieto, J, Thompson, D, Pollmeier, M, Holstes, J (1999) Acceptability of a paste formulation and efficacy of high dose omeprazole in healing gastric ulcers in horses maintained in race training. Equine Vet J Suppl, 29:71-76.
- ↑ Javsicas, L.H, Sanchez, L.C (2008) The effect of omeprazole paste on intragastric pH in clinically ill neonatal foals. Equine Vet J, 40(1):41-4.
- ↑ MacAllister, C.G, Sifferman, R.L, McClure, S.R et al. (1999) Effects of omeprazole paste on healing of spontaneous gastric ulcers in horses and foals: a field trial. Equine Vet J Suppl, 77-80. In: Sanchez, L.C (2010) 'Diseases Of The Stomach' in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 15.
- ↑ Murray, M.J (1994) Gastric ulcers in adult horses. Comp Cont Educ Pract Vet, 16:792-794,797. In: Orsini, J (2000) Tutorial Article Gastric ulceration in the mature horse: a review. Equine Vet Educ, 12(1):24-27.
- ↑ Leandro, G, Pilotto, A, Franceschi, M et al. (2001) Prevention of acute NSAID-related gastroduodenal damage: a meta-analysis fo controlled clinical trials. Dig Dis Sci, 46:1924-1936. In: Sanchez, L.C (2010) 'Diseases Of The Stomach' in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 15.
- ↑ Sangiah, S, MacAllister, C.C, Amouzadeh, H.R (1989) Effects of misoprostol and omeprazole on basal gastric pH and free acid content in horses. Res Vet Sci, 47:350-354. In: Sanchez, L.C (2010) 'Diseases Of The Stomach' in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 15.
- ↑ Tomlinson, J.E, Blikslager, A.T (2005) Effects of cyclooxygenase inhibitors flunixin and deracoxib on permeability of ischaemic-injured equine jejunum. Equine Vet J, 37:75-80. In: Sanchez, L.C (2010) 'Diseases Of The Stomach' in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine (Third Edition), Saunders, Chapter 15.
- ↑ Videla, R, Andrews, F.M (2009) New perspectives in equine gastric ulcer syndrome.Vet Clin North Am Equine Pract, 25(2):283-301.
- ↑ 82.0 82.1 Andrews, F.M, Frank, N, Sommardahl, C.S, Buchanan, B.R, Elliott, S.B, Allen, V.A (2006) Effects of intravenously administrated omeprazole on gastric juice pH and gastric ulcer scores in adult horses. J Vet Intern Med, 20(5):1202-6.