Pulpy Kidney
Also known as: Clostridium perfringens type D enterotoxaemia
Introduction
Pulpy kidney is a common, peracute and usually fatal enterotoxaemia of sheep of all ages, caused by the ε toxin of Clostridium perfringens type D. Clostridium perfringens type D causes the highest number of sheep fatalities due to clostridial disease1. It is a large, gram positive, anaerobic bacillus that is ubiquitous in the environment and commensalises the gastrointestinal tract of most mammals, although type D is found less abundantly in healthy animals than the other genotypes2. Five genotypes of Clostridium perfringens exist, named A-E, and all genotypes produce potent exotoxins. There are 12 exotoxins in total, some of which are lethal and others which are of minor significance3. These are produced as pro-toxins, and are converted to their toxic forms by digestive enzymes such as trypsin. The enterotoxaemias are a group of diseases caused by proliferation of C. perfringens in the lumen of the gastrointestinal tract and excessive production of exotoxin.
In healthy animals, there is a balance between multiplication of Clostridium perfringens and its passage in the faeces. This ensures that infection is maintained at a low level. However, C. perfringens is saccharolytic and is therefore able to multiply rapidly when large quantities of fermentable carbohydrate are introduced to the anaerobic conditions of the abomasum and small intestine, leading to build-up of exotoxin. Gut statis, for example due to insufficient dietary fibre or a high gastrointestinal parasite burden, can also contribute to the accumulation of toxins.
Enterotoxaemia due to Clostridium perfringens type D causes sudden death in sheep of any age, particularly well-grown lambs of between 4 and 10 weeks of age and fattening lambs of 6 months to 1 year old3. Rams are also susceptible when they are subjected to an increased plane of nutrition prior to mating. The condition is associated with a change in diet, for example the introdcution of lush grass or high proportions of concentrate. This leads to rapid multiplication of the bacterium and excessive production of its ε toxin. ε toxin causes loss of epithelial integrity, increasing the permeability of the intestinal mucosa to facilitate its rapid absorption1. It then gives degeneration of vascular endothelium elsewhere, particularly in the brain, leading to increased capiliary permeability and oedema formation2, 4. Hepatic glycogen is mobilised by ε toxin and so hyperglycaemia and glycosuria are frequently seen1.Endothelial damage results in protein-rich effusions. The incidence of pulpy kidney declined over the past 25 years due to the widespread use of clostridial vaccines5, but the condition is becoming a problem again as complacency reduces the use of vaccination. At its most extreme, pulpy kidney can cause losses of 10-15% of the lamb crop. The disease can also occur in cattle, but this is rare2.
Signalment
Pulpy kidney can affect unvaccinated sheep of all ages. However, it is most common in 4-10 week old lambs, and fattening lambs between 6 months and 1 year of age.
Diagnosis
A presumptive diagnosis of pulpy kidney can be made on the basis a history of sudden deaths in lambs recently introduced to carbohydrate-rich feed. If animals are seen before they die, certain clinical signs may be suggestive of pulpy kidney, and post-mortem examination may also aid diagnosis. However, histopathology of the brain is the most useful diagnostic test.
Clinical Signs
The first indication of pulpy kidney disease is the occurence of sudden death in the best-grown lambs1-4. Occasionally, animals may be seen alive displaying clinical signs suggestive of the condition. Signs are typically neurological and include hyperaesthesia, ataxia, circling or head-pressing, with rapid progression to recumbency, opisthotonus, convulsions and death1-4. Diarrhoea may also be seen, and hyperglycemia or glycosuria is frequently reported1-4.
Adult sheep are sometimes affected and show weakness, incoordination, and convulsions. Death occurs within 24 hours4.
Laboratory Tests
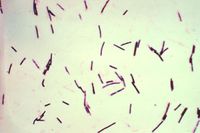
Short, thick gram-positive rods are easily visualised on smears of intestinal contents. Culture under anaerobic conditions usually gives pure cultures of C. perfringens, which can be demonstrated as being type D by PCR techniques. An ELISA may be used to demonstrate the presence of ɛ toxin in the small intestinal or peritoneal fluid. In contrast to lamb dysentery, in pulpy kidney ε toxin will be demonstrated in the absence of β toxin1. A positive toxin ELISA supports but does not confirm a diagnosis, since immune animals may experience elevated toxin levels without suffering ill effects.
Pathology
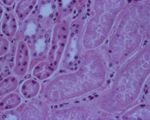
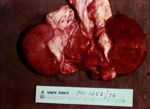
In young lambs, the only change observable on post-mortem examination may be the presence of a few hyperaemic areas on the intestine and a fluid-filled pericardial sac4. Intestinal lesions may even be absent2. Animals, particularly older ones, may have myocardial haemorrhages as well as petechiation of visceral surfaces and abdominal muscles2, 4, 6. Pulmonary oedema and congestion is often present and there may be pleural, peritoneal or pericardial effusions2, 4. The fluid is variable in volume and can be straw or red coloured, sometimes containing fibrin clotsivis. The kidneys often rapidly autolyse, as suggested by the name "pulpy kidney", but this is not a pathognomic finding.
Lesions of the central nervous system account for the neurologic signs. There is a bilaterally symmetrical focal malacia of the basal ganglia and thalamus, with demyelination occuring in the internal capsule, subcortical white matter and cerebellar peduncles6. Histological examination of the brain is often used to confirm a diagnosis of pulpy kidney1. Capillaries are occluded by aggregated platelets, and grossly this gives petechiation of the damaged areas. The cerebellar white matter shows periaxonal and intramyelinic oedema, and there is swelling of axon terminals and dendrites near the lateral ventricles6. The nuceli of damaged endothelial cells appear pyknotic, and astrocyte end feet are markedly swollen. Swelling of mitochondria may also be seen 6.
Treatment and Control
The first sign of pulpy kidney is sudden death, and so there is often no opportunity for treatment. Even if affected animals are found prior to death, treatment is usually unrewarding as organs are irreversibly damaged by toxins by the time signs present3. Instead, a definitive diagnosis should be pursued before greater losses occur, and the farmer should be encouraged to submit the carcase for further investigations.
Control of pulpy kidney involves avoiding the factors that can precipitate disease. Diets should not be changed suddenly and concentrate feeding should always be introduced slowly1. Feeding of whole grain slows the transit of feed to the small intestine, so cereals should be fed in this form. C.. perfringens type D is ubiquitously distributed and so management measure will never completely prevent disease. The best method of disease prevention is vaccination.
Before the development of modern clostridial vaccines in the 1970s, losses of up to 15% of the lamb crop could occur due to pulpy kidney3. The vaccines used today are effective against a variety of clostridial diseases and some vaccines are combined for effects against Pasteurella. The vaccines consist of toxoids which are inactivated forms of the toxins produced by clostridial organisms. The principles of vaccination are the same whether a clostridium-only or Pasteurella-combined product is used: a sensitising dose must be given 4-6 weeks before a second, confirming dose3. As immunity wanes over a period of a year, booster doses are required annually. Therefore, ewes should receive the primary vaccination course before entering the breeding flock and an annual booster approximately six weeks before lambing. Timing the booster vaccination in this way affords passive protection to lambs until they are around 16 weeks of age. Lambs born to unvaccinated ewes should be vaccinated between 3 and 12 weeks old, with a second injection given at least four weeks later.
| Pulpy Kidney Learning Resources | |
|---|---|
 Search for recent publications via CAB Abstract (CABI log in required) |
Pulpy Kidney publications |
Links
- The Merck Veterinary Manual: Pulpy Kidney
- The Center for Food Security and Public Health Animal Disease Factsheet: Epsilon toxin of Clostridium perfringens.
- NOAH: Vaccination of farm animals
- Clostridia.net - Clostridium perfringens
References
- Aitken, I D (2007) Diseases of sheep, Wiley-Blackwell.
- Van Metre (2006) Clostridial Infections of the Ruminant GI Tract. Proceedings of the North American Veterinary Conference 2006.
- Lewis, C (1998) Aspects of clostridial disease in sheep. In Practice, 20(9), 494-499.
- Merck & Co (2008) The Merck Veterinary Manual (Eighth Edition), Merial.
- Sargison, N (2004) Differential diagnosis of diarrhoea in lambs. In Practice, 26(1), 20-27.
- Jones, T C et al (1997) Veterinary Pathology, Wiley-Blackwell.
- Songer, J G (1998) Clostridial diseases of small ruminants. Veterinary Research, 29, 219-232.
- The Center for Food Security and Public Health, Iowa State University (2004) Animal Disease Factsheet: Epsilon toxin of Clostridium perfringens.
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
Webinars
Failed to load RSS feed from https://www.thewebinarvet.com/urogenital-and-reproduction/webinars/feed: Error parsing XML for RSS