Difference between revisions of "Preparation and Examination of Blood Smear"
Fiorecastro (talk | contribs) |
|||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:NationWide Logo.jpeg|right|link=https://www.nwlabs.co.uk/|alt=NationWide Logo|240x240px|In Partnership with NationWide Laboratories|frameless|thumb|]] | [[File:NationWide Logo.jpeg|right|link=https://www.nwlabs.co.uk/|alt=NationWide Logo|240x240px|In Partnership with NationWide Laboratories|frameless|thumb|]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Preparation of Blood Smear == | == Preparation of Blood Smear == | ||
| Line 15: | Line 8: | ||
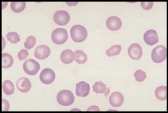
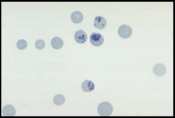
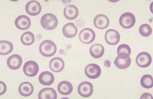
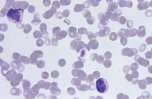
Well prepared blood smears should have an area where the blood cells are in a monolayer. Place a small drop of blood on the slide towards one end. Draw the spreader slide back to contact the drop and then move it forwards before the blood has spread along the width of the spreader slide. This will prevent the smear extending to the extremities of the slide, when cells can be lost over the edges. The smear should terminate before the end of the slide (Fig 1). | Well prepared blood smears should have an area where the blood cells are in a monolayer. Place a small drop of blood on the slide towards one end. Draw the spreader slide back to contact the drop and then move it forwards before the blood has spread along the width of the spreader slide. This will prevent the smear extending to the extremities of the slide, when cells can be lost over the edges. The smear should terminate before the end of the slide (Fig 1). | ||
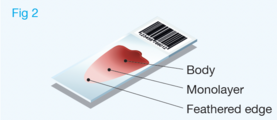
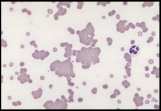
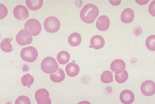
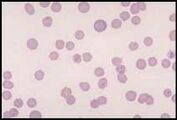
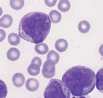
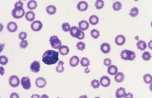
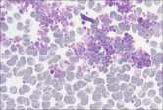
The area to be examined is the monolayer where 50% or less of cells are in contact. The differential count is performed, and cell morphology is assessed in the monolayer (Fig 2). | The area to be examined is the monolayer where 50% or less of cells are in contact. The differential count is performed, and cell morphology is assessed in the monolayer (Fig 2). | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:Nation Wide Laboratories.png | + | File:Nation Wide Laboratories.png |
File:NationWide Laboratories Fig2.png | File:NationWide Laboratories Fig2.png | ||
</gallery> | </gallery> | ||
| Line 119: | Line 112: | ||
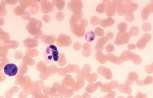
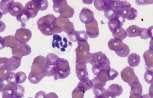
<u>'''Artefacts'''.</u> Stain precipitate, refractile bodies and echinocytes. | <u>'''Artefacts'''.</u> Stain precipitate, refractile bodies and echinocytes. | ||
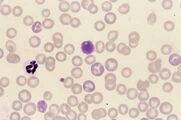
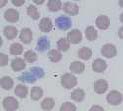
Echinocytes (crenated red cells). Red cells with many spiny projections which tend to be evenly spaced and uniform. This is often artefact seen in thick smears, which have taken a long time to dry, or due to excess EDTA, if the tube is not filled to the line. Echinocytes can be associated with pathological changes such as uraemia, glomerulonephritis, neoplasia and snake venom. | Echinocytes (crenated red cells). Red cells with many spiny projections which tend to be evenly spaced and uniform. This is often artefact seen in thick smears, which have taken a long time to dry, or due to excess EDTA, if the tube is not filled to the line. Echinocytes can be associated with pathological changes such as uraemia, glomerulonephritis, neoplasia and snake venom. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWL Labfacts .jpg| | + | File:NWL Labfacts .jpg|Stain precipitate NationWide Laboratories |
| − | File:NWL Lab.jpg| | + | File:NWL Lab.jpg|Refractile bodies NationWide Laboratories |
| − | File:NWLab .jpg| | + | File:NWLab .jpg|Echinocytes, a small and large platelet NationWide Laboratories|alt= |
</gallery> | </gallery> | ||
| Line 129: | Line 122: | ||
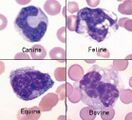
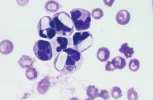
<u>'''Anisocytosis.'''</u> Refers to variation in RBC size. Immature cells (reticulocytes) are larger than mature erythrocytes. | <u>'''Anisocytosis.'''</u> Refers to variation in RBC size. Immature cells (reticulocytes) are larger than mature erythrocytes. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWL 2016 Labfacts inners A.jpg| | + | File:NWL 2016 Labfacts inners A.jpg|Anisocytosis and Polychromasia NationWide Laboratories |
</gallery> | </gallery> | ||
| Line 157: | Line 150: | ||
|} | |} | ||
The higher the grade, the more marked the degree of regeneration in response to anaemia. Anisocytosis and polychromasia in non-anaemic patients may reflect poor oxygenation of blood due to cardiac or respiratory disease. | The higher the grade, the more marked the degree of regeneration in response to anaemia. Anisocytosis and polychromasia in non-anaemic patients may reflect poor oxygenation of blood due to cardiac or respiratory disease. | ||
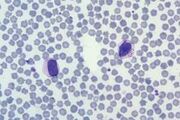
| − | <br/><u>'''Reticulocytes'''</u>. Immature red cells, their residual RNA and mitochondria stain with new methylene blue. | + | <br/> |
| − | <gallery widths= | + | <u>'''Reticulocytes'''</u>. Immature red cells, their residual RNA and mitochondria stain with new methylene blue. |
| − | File:NWL 2016.jpg| | + | <gallery widths=300> |
| − | File:NW laboratories.jpg| | + | File:NWL 2016.jpg|Canine reticulocytes NationWide Laboratories|alt= |
| + | File:NW laboratories.jpg|Feline Reticulocytes NationWide Laboratories | ||
</gallery> | </gallery> | ||
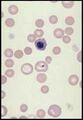
<u>'''Nucleated red blood cells (metarubricytes)'''.</u> These are immature red cells, which retain a condensed nucleus. | <u>'''Nucleated red blood cells (metarubricytes)'''.</u> These are immature red cells, which retain a condensed nucleus. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NationWide Lab 2016.jpg| | + | File:NationWide Lab 2016.jpg|Nucleated red blood cell NationWide Laboratories |
</gallery> | </gallery> | ||
=== Immune mediated haemolytic anaemia (IMHA) === | === Immune mediated haemolytic anaemia (IMHA) === | ||
<u>'''Agglutination'''.</u> This describes the random, three dimensional clumping of red cells. Autoagglutination is persistent agglutination, which cannot be dispersed in saline. It is pathognomonic for IMHA but is not seen in all cases. Autoagglutination will affect red cell indices in automated analysers (increased MCV, MCH and MCHC). | <u>'''Agglutination'''.</u> This describes the random, three dimensional clumping of red cells. Autoagglutination is persistent agglutination, which cannot be dispersed in saline. It is pathognomonic for IMHA but is not seen in all cases. Autoagglutination will affect red cell indices in automated analysers (increased MCV, MCH and MCHC). | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 1.jpg| | + | File:NWLLabfacts 1.jpg|Autoagglutination NationWide Laboratories |
</gallery> | </gallery> | ||
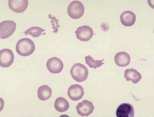
<u>'''Spherocytes''':</u> These are spherical erythrocytes which appear smaller in diameter, stain more densely and lack central pallor. They are more obvious in canine blood (feline erythrocytes naturally lack central pallor) and are most commonly associated with IMHA. Other causes include snake bite, zinc toxicity and bee stings. | <u>'''Spherocytes''':</u> These are spherical erythrocytes which appear smaller in diameter, stain more densely and lack central pallor. They are more obvious in canine blood (feline erythrocytes naturally lack central pallor) and are most commonly associated with IMHA. Other causes include snake bite, zinc toxicity and bee stings. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 2.jpg| | + | File:NWLLabfacts 2.jpg|Spherocytes with central polychromatophil NationWide Laboratories |
</gallery> | </gallery> | ||
| Line 184: | Line 178: | ||
=== Other morphological changes === | === Other morphological changes === | ||
<u>'''Acanthocytes'''</u>. Erythrocytes with a few irregular, asymmetrical projections. In dogs acanthocytes may be associated with haemangiosarcoma, lymphoma, DIC and altered lipid metabolism (hepatic disease). In cats they may be recognised with liver disease including the cholangitis complex and hepatic lipidosis. | <u>'''Acanthocytes'''</u>. Erythrocytes with a few irregular, asymmetrical projections. In dogs acanthocytes may be associated with haemangiosarcoma, lymphoma, DIC and altered lipid metabolism (hepatic disease). In cats they may be recognised with liver disease including the cholangitis complex and hepatic lipidosis. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 3.jpg| | + | File:NWLLabfacts 3.jpg|Acanthocytes NationWide Laboratories |
</gallery> | </gallery> | ||
<u>'''Basophilic stippling'''.</u> Reflects the presence of aggregated ribosomal RNA in reticulocytes and is primarily associated with regenerative anaemias. It is also evident with the exaggerated regenerative response seen in many cases of lead poisoning (often in the absence of anaemia), due to the effect of lead on the bone marrow. | <u>'''Basophilic stippling'''.</u> Reflects the presence of aggregated ribosomal RNA in reticulocytes and is primarily associated with regenerative anaemias. It is also evident with the exaggerated regenerative response seen in many cases of lead poisoning (often in the absence of anaemia), due to the effect of lead on the bone marrow. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 4.jpg| | + | File:NWLLabfacts 4.jpg|Basophilic stippling NationWide Laboratories |
</gallery> | </gallery> | ||
| Line 196: | Line 190: | ||
<u>'''Heinz bodies'''</u>. Oxidation of sulphydryl groups on the globin chains of haemoglobin results in the formation of Heinz bodies. These are refractile structures which occur along the internal surface of erythrocyte membranes, seen as pale pink-red projections with Romanowsky stains. Normal cats may have up to 5% Heinz bodies in their erythrocytes. Heinz body formation in the absence of anaemia may occur with feline diabetes mellitus, lymphoma and hyperthyroidism. Increased numbers of Heinz bodies, leading to haemolytic anaemia, may be associated with onion or garlic ingestion, kale or other brassica species consumption by ruminants and consumption of red maple leaves by alpacas and horses. | <u>'''Heinz bodies'''</u>. Oxidation of sulphydryl groups on the globin chains of haemoglobin results in the formation of Heinz bodies. These are refractile structures which occur along the internal surface of erythrocyte membranes, seen as pale pink-red projections with Romanowsky stains. Normal cats may have up to 5% Heinz bodies in their erythrocytes. Heinz body formation in the absence of anaemia may occur with feline diabetes mellitus, lymphoma and hyperthyroidism. Increased numbers of Heinz bodies, leading to haemolytic anaemia, may be associated with onion or garlic ingestion, kale or other brassica species consumption by ruminants and consumption of red maple leaves by alpacas and horses. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 5.jpg| | + | File:NWLLabfacts 5.jpg|Heinz Bodies NationWide Laboratories |alt= |
</gallery> | </gallery> | ||
| Line 204: | Line 198: | ||
<u>'''Howell-Jolly bodies.'''</u> These are remnants of nuclear material . They are present in regenerative anaemias possibly due to inability of macrophages to fully remove the nuclei of maturing RBCs during accelerated production. If present without polychromasia, reduced macrophage function could be considered. They may be a common finding following splenectomy. | <u>'''Howell-Jolly bodies.'''</u> These are remnants of nuclear material . They are present in regenerative anaemias possibly due to inability of macrophages to fully remove the nuclei of maturing RBCs during accelerated production. If present without polychromasia, reduced macrophage function could be considered. They may be a common finding following splenectomy. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 6.jpg| | + | File:NWLLabfacts 6.jpg|Howell-Jolly bodies NationWide Laboratories|alt= |
</gallery> | </gallery> | ||
| Line 212: | Line 206: | ||
'''<u>Red cell fragmentation.</u>''' Small fragments are called schistocytes (schizocytes), larger red cell fragments include keratocytes (blister and helmet cells). These reflect metabolic disease or, more commonly, intravascular trauma associated with turbulent blood flow due to cardiac valvular disease, with passage through numerous capillaries in haemangiosarcoma and certain other malignant neoplasms, or fibrin strands cleaving erythrocytes in myelofibrosis or DIC. Severe hepatic disease may also result in red cell fragmentation. | '''<u>Red cell fragmentation.</u>''' Small fragments are called schistocytes (schizocytes), larger red cell fragments include keratocytes (blister and helmet cells). These reflect metabolic disease or, more commonly, intravascular trauma associated with turbulent blood flow due to cardiac valvular disease, with passage through numerous capillaries in haemangiosarcoma and certain other malignant neoplasms, or fibrin strands cleaving erythrocytes in myelofibrosis or DIC. Severe hepatic disease may also result in red cell fragmentation. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 8.jpg| | + | File:NWLLabfacts 8.jpg|Red Cell fragmentation NationWide Laboratories |
</gallery> | </gallery> | ||
| Line 219: | Line 213: | ||
'''<u>Rouleaux.</u>''' These are stacks of erythrocytes resembling a pile of coins, which disperse in saline. Rouleaux are most obvious when examining the body of the blood smear. | '''<u>Rouleaux.</u>''' These are stacks of erythrocytes resembling a pile of coins, which disperse in saline. Rouleaux are most obvious when examining the body of the blood smear. | ||
Rouleaux are prominent in blood smears from normal horses and some cats. Rouleaux are alsoseen in animals with hyperglobulinaemia, particularly h yperfibrinogenaemia and elevated acute phase proteins. | Rouleaux are prominent in blood smears from normal horses and some cats. Rouleaux are alsoseen in animals with hyperglobulinaemia, particularly h yperfibrinogenaemia and elevated acute phase proteins. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 9.jpg| | + | File:NWLLabfacts 9.jpg|Rouleaux in an equine blood smear NationWide Laboratories |
</gallery> | </gallery> | ||
'''<u>Target cells (codocytes)</u>'''. These are bell-shaped in-vivo but resemble a target on a blood smear due to accumulation of haemoglobin around the periphery and centre. | '''<u>Target cells (codocytes)</u>'''. These are bell-shaped in-vivo but resemble a target on a blood smear due to accumulation of haemoglobin around the periphery and centre. | ||
They may be encountered with regenerative anaemia, renal, hepatic or lipid disorders. | They may be encountered with regenerative anaemia, renal, hepatic or lipid disorders. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 10.jpg| | + | File:NWLLabfacts 10.jpg|Target Cells (codocytes) NationWide Laboratories|alt= |
</gallery> | </gallery> | ||
=== Inclusions === | === Inclusions === | ||
Mycoplasma haemofelis (see image) | Mycoplasma haemofelis (see image) | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 11.jpg| | + | File:NWLLabfacts 11.jpg|Mycoplasma haemofelis NationWide Laboratories|alt= |
</gallery> | </gallery> | ||
Babesia canis (see image) | Babesia canis (see image) | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 12.jpg| | + | File:NWLLabfacts 12.jpg|Babesia Canis NationWide Laboratories |
</gallery> | </gallery> | ||
| Line 244: | Line 238: | ||
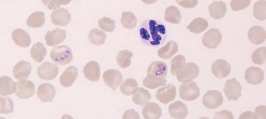
'''<u>Neutrophil.</u>''' A mature neutrophil has a segmented nucleus with condensed chromatin and fine intracytoplasmic azurophilic granules. | '''<u>Neutrophil.</u>''' A mature neutrophil has a segmented nucleus with condensed chromatin and fine intracytoplasmic azurophilic granules. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 13.jpg| | + | File:NWLLabfacts 13.jpg|Neutrophil NationWide Laboratories |
</gallery> | </gallery> | ||
In females, the nucleus may have a drum-stick shaped appendage known as a Barr body which represents the inactive X chromosome; this is of no clinical significance. | In females, the nucleus may have a drum-stick shaped appendage known as a Barr body which represents the inactive X chromosome; this is of no clinical significance. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 14.jpg| | + | File:NWLLabfacts 14.jpg|Barr body NationWide Laboratories |
</gallery> | </gallery> | ||
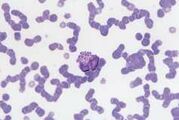
'''<u>Band neutrophil.</u>''' This is an immature cell with an unsegmented nucleus showing parallel sides.The cytoplasm is similar to that of a mature cell. | '''<u>Band neutrophil.</u>''' This is an immature cell with an unsegmented nucleus showing parallel sides.The cytoplasm is similar to that of a mature cell. | ||
Sometimes less differentiated myeloid cells (metamyelocytes and myelocytes) are seen in the blood, usually associated with a severe acute inflammatory response. This is called a left shift. | Sometimes less differentiated myeloid cells (metamyelocytes and myelocytes) are seen in the blood, usually associated with a severe acute inflammatory response. This is called a left shift. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 16.jpg| | + | File:NWLLabfacts 16.jpg|Metamyelocyte NationWide Laboratories |
</gallery> | </gallery> | ||
'''<u>Toxic neutrophil.</u>''' Toxic change describes the leucocyte morphological abnormalities, seen predominantly in neutrophils, in the face of severe inflammation. The cytoplasm may show increased basophilia (light blue or grey rather than pink) and may contain Dohle bodies, which are small round or angular blue inclusions representing retained RNA (normally degraded during maturation). Dohle bodies are often seen in feline neutrophils and are not clinically significant unless frequent and prominent. | '''<u>Toxic neutrophil.</u>''' Toxic change describes the leucocyte morphological abnormalities, seen predominantly in neutrophils, in the face of severe inflammation. The cytoplasm may show increased basophilia (light blue or grey rather than pink) and may contain Dohle bodies, which are small round or angular blue inclusions representing retained RNA (normally degraded during maturation). Dohle bodies are often seen in feline neutrophils and are not clinically significant unless frequent and prominent. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 17.jpg| | + | File:NWLLabfacts 17.jpg|Dohle bodies in the cytoplasm of a neutrophil NationWide Laboratories |
</gallery> | </gallery> | ||
Neutrophils may have swollen nuclei which sometimes form doughnuts. | Neutrophils may have swollen nuclei which sometimes form doughnuts. | ||
Toxic change occurs in the bone marrow and reflects accelerated granulopoiesis, especiallyassociated with bacterial infections and IMHA. | Toxic change occurs in the bone marrow and reflects accelerated granulopoiesis, especiallyassociated with bacterial infections and IMHA. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 18.jpg| | + | File:NWLLabfacts 18.jpg|thumb|Neutrophil with toxic granulation NationWide Laboratories |
</gallery> | </gallery> | ||
There may be toxic granulation due to staining of primary granules. | There may be toxic granulation due to staining of primary granules. | ||
In severe toxaemia, neutrophils may show cytoplasmic vacuolation due to degranulation of lysosomes during disturbed maturation. | In severe toxaemia, neutrophils may show cytoplasmic vacuolation due to degranulation of lysosomes during disturbed maturation. | ||
| − | '''<u>Hypersegmented neutrophil.</u>''' There are more than 5 nuclear lobes. Found in dogs on corticosteroid therapy (which prolongs the half life of the neutrophil) and may be associated with uraemia. Also seen in Poodles with familial macrocytosis. <gallery widths= | + | |
| − | File:NWLLabfacts 19.jpg| | + | '''<u>Hypersegmented neutrophil.</u>''' There are more than 5 nuclear lobes. Found in dogs on corticosteroid therapy (which prolongs the half life of the neutrophil) and may be associated with uraemia. Also seen in Poodles with familial macrocytosis. |
| + | <gallery widths=300px> | ||
| + | File:NWLLabfacts 19.jpg|Hypersegmented neutrophil NationWide Laboratories | ||
</gallery> | </gallery> | ||
| Line 280: | Line 276: | ||
'''<u>Eosinophil.</u>''' Mature eosinophils have a segmented nucleus and red intracytoplasmic granules. The granules in the dog are round and vary in size. In the cat they are small, uniform in size and rod shaped while in the horse the granules are round and very large. In rabbits, eosinophils have larger red granules than neutrophils although often the two cell types are difficult to differentiate and may be counted together as heterophils. Band eosinophils are immature cells showing less distinct nuclear segmentation. | '''<u>Eosinophil.</u>''' Mature eosinophils have a segmented nucleus and red intracytoplasmic granules. The granules in the dog are round and vary in size. In the cat they are small, uniform in size and rod shaped while in the horse the granules are round and very large. In rabbits, eosinophils have larger red granules than neutrophils although often the two cell types are difficult to differentiate and may be counted together as heterophils. Band eosinophils are immature cells showing less distinct nuclear segmentation. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 20.jpg| | + | File:NWLLabfacts 20.jpg|Equine eosinophil NationWide Laboratories |
</gallery> | </gallery> | ||
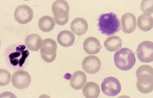
'''<u>Basophil.</u>''' Rare in blood smears from healthy animals. Basophils are similar in size to eosinophils, with a segmented, ribbon-like nucleus and variable numbers of cytoplasmic granules. In dogs these are deep purple and sparse. Cats have more numerous lilac coloured granules with occasional deep purple granules. | '''<u>Basophil.</u>''' Rare in blood smears from healthy animals. Basophils are similar in size to eosinophils, with a segmented, ribbon-like nucleus and variable numbers of cytoplasmic granules. In dogs these are deep purple and sparse. Cats have more numerous lilac coloured granules with occasional deep purple granules. | ||
| − | <gallery widths= | + | <gallery widths=300px> |
| − | File:NWLLabfacts 21.jpg| | + | File:NWLLabfacts 21.jpg|Basophil NationWide Laboratories |
</gallery> | </gallery> | ||
'''<u>Mast cell.</u>''' These are not present in the blood of healthy animals but may be present with severe inflammatory disease or metastatic mast cell tumours (mastocytaemia). They are round cells with a round nucleus surrounded by a moderate volume of cytoplasm containing large numbers of purple granules. | '''<u>Mast cell.</u>''' These are not present in the blood of healthy animals but may be present with severe inflammatory disease or metastatic mast cell tumours (mastocytaemia). They are round cells with a round nucleus surrounded by a moderate volume of cytoplasm containing large numbers of purple granules. | ||
<gallery widths="300"> | <gallery widths="300"> | ||
| − | File:NWLLabfacts 22.jpg| | + | File:NWLLabfacts 22.jpg|Mast cell NationWide Laboratories |
</gallery> | </gallery> | ||
| Line 297: | Line 293: | ||
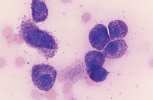
'''<u>Reactive lymphocyte.</u>''' Slightly larger than a mature lymphocyte. Cells have round nuclei with condensed nuclear chromatin, surrounded by a rim of pale basophilic cytoplasm. | '''<u>Reactive lymphocyte.</u>''' Slightly larger than a mature lymphocyte. Cells have round nuclei with condensed nuclear chromatin, surrounded by a rim of pale basophilic cytoplasm. | ||
<gallery widths="300"> | <gallery widths="300"> | ||
| − | File:NWLLabfacts 23.jpg| | + | File:NWLLabfacts 23.jpg|Small, medium and large Lymphocytes NationWide Laboratories |
</gallery> | </gallery> | ||
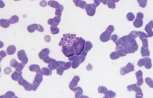
<u>'''Lymphoblast.'''</u> A large cell with a large nucleus, two to three red cell widths in diameter, with one or more prominent nucleoli. These are rarely seen in blood smears from healthy animals but may be present with lymphoid neoplasia. | <u>'''Lymphoblast.'''</u> A large cell with a large nucleus, two to three red cell widths in diameter, with one or more prominent nucleoli. These are rarely seen in blood smears from healthy animals but may be present with lymphoid neoplasia. | ||
<gallery widths="300"> | <gallery widths="300"> | ||
| − | File:NWLLabfacts 24.jpg| | + | File:NWLLabfacts 24.jpg|Lymphoblast NationWide Laboratories |
</gallery> | </gallery> | ||
| Line 309: | Line 305: | ||
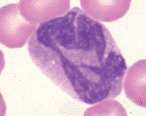
'''<u>Large granular lymphocyte</u>'''. Lymphocyte containing several prominent magenta coloured cytoplasmic granules. Rarely seen in peripheral blood but are numerous in large granular cell leukaemia in the dog | '''<u>Large granular lymphocyte</u>'''. Lymphocyte containing several prominent magenta coloured cytoplasmic granules. Rarely seen in peripheral blood but are numerous in large granular cell leukaemia in the dog | ||
<gallery widths="300"> | <gallery widths="300"> | ||
| − | File:NWLLabfacts 25.jpg| | + | File:NWLLabfacts 25.jpg|Large granular Lymphocyte NationWide Laboratories |
</gallery> | </gallery> | ||
<u>'''Monocyte'''</u>. These are large cells with a deeply indented nucleus showing pale, finely granular nuclear chromatin. They have a moderate amount of blue-grey cytoplasm, which may contain vacuoles. | <u>'''Monocyte'''</u>. These are large cells with a deeply indented nucleus showing pale, finely granular nuclear chromatin. They have a moderate amount of blue-grey cytoplasm, which may contain vacuoles. | ||
<gallery widths="300"> | <gallery widths="300"> | ||
| − | File:NWLLabfacts 26.jpg| | + | File:NWLLabfacts 26.jpg|Monocyte NationWide Laboratories |
</gallery> | </gallery> | ||
| Line 320: | Line 316: | ||
Canine distemper intracytoplasmic inclusion in a neutrophil (See image) | Canine distemper intracytoplasmic inclusion in a neutrophil (See image) | ||
<gallery widths="300"> | <gallery widths="300"> | ||
| − | File:NWLLabfacts 27.jpg| | + | File:NWLLabfacts 27.jpg|Canine distemper intracytoplasmic inclusion in a neutrophil NationWide Laboratories |
</gallery> | </gallery> | ||
Ehrlichia canis morula in a monocyte (see image) | Ehrlichia canis morula in a monocyte (see image) | ||
<gallery widths="300"> | <gallery widths="300"> | ||
| − | File:NWL 1.jpg| | + | File:NWL 1.jpg|Ehrlichia canis morula in a monocyte NationWide Laboratories |
</gallery> | </gallery> | ||
Leishmania in the cytoplasm of a neutrophil (see image) | Leishmania in the cytoplasm of a neutrophil (see image) | ||
<gallery widths="300"> | <gallery widths="300"> | ||
| − | File:NWL 2.jpg| | + | File:NWL 2.jpg|Leishmania in the cytoplasm of neutrophil NationWide Laboratories |
</gallery> | </gallery> | ||
Anaplasma platys elementary bodies (see image) | Anaplasma platys elementary bodies (see image) | ||
<gallery widths="300"> | <gallery widths="300"> | ||
| − | File:NWL 3.jpg| | + | File:NWL 3.jpg|Anaplasma platys elementary bodies NationWide Laboratories |
</gallery> | </gallery> | ||
Hepatozoon gametocytes and Ehrlichia Morula (see image) | Hepatozoon gametocytes and Ehrlichia Morula (see image) | ||
<gallery widths="300"> | <gallery widths="300"> | ||
| − | File:NWL 4.jpg| | + | File:NWL 4.jpg|Hepatozoon gametocytes and Ehrlichia Morula NationWide Laboratories |
</gallery> | </gallery> | ||
| Line 347: | Line 343: | ||
Platelet numbers can be estimated from the blood smear as described previously. The smear should be checked for any platelet clumps which are often found in the tail (feathered edge) of the smear. Clumping of platelets is frequently seen in cats and results in a spuriously low platelet count. | Platelet numbers can be estimated from the blood smear as described previously. The smear should be checked for any platelet clumps which are often found in the tail (feathered edge) of the smear. Clumping of platelets is frequently seen in cats and results in a spuriously low platelet count. | ||
<gallery widths="300"> | <gallery widths="300"> | ||
| − | File:NWL 5.jpg| | + | File:NWL 5.jpg|Clumps of platelets, often found in the feathered edge NationWide Laboratories |
</gallery> | </gallery> | ||
'''<u>Macrothrombocyte.</u>''' A large platelet indicating enhanced thrombopoiesis. Larger platelets are more functionally active and this may explain why some dogs with thrombocytopaenia, but largeplatelets, do not bleed. | '''<u>Macrothrombocyte.</u>''' A large platelet indicating enhanced thrombopoiesis. Larger platelets are more functionally active and this may explain why some dogs with thrombocytopaenia, but largeplatelets, do not bleed. | ||
<gallery widths="300"> | <gallery widths="300"> | ||
| − | File:NWL 6.jpg| | + | File:NWL 6.jpg|Macrothrombocyte Nation Wide Laboratories (the one in the middle) |
</gallery> | </gallery> | ||
Primary platelet disorders such as thrombasthenic thrombopathia of otter hounds are rare, producing large and morphologically bizarre platelets. Cavalier King Charles spaniels may have large platelets with decreased platelet counts due to inherited macrothrombocytopaenia. | Primary platelet disorders such as thrombasthenic thrombopathia of otter hounds are rare, producing large and morphologically bizarre platelets. Cavalier King Charles spaniels may have large platelets with decreased platelet counts due to inherited macrothrombocytopaenia. | ||
<gallery widths="300"> | <gallery widths="300"> | ||
| − | File:NWL 7.jpg| | + | File:NWL 7.jpg|Inherited macrothrombocytopaenia NationWide Laboratories |
</gallery> | </gallery> | ||
== Authors and References == | == Authors and References == | ||
| − | [[NationWide Laboratories]] | + | [[NationWide Laboratories#In Partnership with NationWide Laboratories]] |
[[Category:Pathological Sample Collection|5]] | [[Category:Pathological Sample Collection|5]] | ||
| − | |||
Revision as of 14:09, 3 March 2022
Preparation of Blood Smear
Blood smears should be prepared immediately to avoid artefact due to exposure to anticoagulant. Smears prepared from an EDTA sample can be used for a routine differential count but the cell morphology is not ideal. Well prepared fresh smears are particularly important for examining atypical cell morphology.
If parasitaemia is a concern, smears should be made from capillary blood (ear prick) since the concentration of parasites is higher in peripheral blood.
Well prepared blood smears should have an area where the blood cells are in a monolayer. Place a small drop of blood on the slide towards one end. Draw the spreader slide back to contact the drop and then move it forwards before the blood has spread along the width of the spreader slide. This will prevent the smear extending to the extremities of the slide, when cells can be lost over the edges. The smear should terminate before the end of the slide (Fig 1). The area to be examined is the monolayer where 50% or less of cells are in contact. The differential count is performed, and cell morphology is assessed in the monolayer (Fig 2).
The Complete Blood Count
This is a profile of tests to determine the quantity and quality of blood cells. It is usually performed on a haematology analyser, either a large commercial analyser used by an external diagnostic laboratory or a smaller in clinic analyser.
The CBC includes:
- Red cell count
- Haemoglobin concentration
- PCV/Haematocrit
- Mean corpuscular volume (MCV)
- Mean corpuscular haemoglobin concentration (MCHC)
- White blood cell count
- Differential cell count
- Nucleated RBC
- Undifferentiated cells
- Platelet count
Red Cell Count and White Cell Count
These can be obtained using an automated cell counter or manually, using a haemocytometer. For exotic and avian species with nucleated red cells, manual counts are essential.
The numbers of cells are quantified: RBC x 1012/l and WBC x 109/l.
The total WBC count should be corrected for the presence of nucleated red blood cells (NRBC) since these will be counted as WBCs.
Corrected WBC count = 100 / (NRBC + 100) x nucleated cell count.
The WBC count can be estimated from the buffy coat of the microhaematocrit tube (see over). The first 1% of the buffy coat represents approximately 10x109 cells/l and each subsequent 1% represents an additional 20 x109 cells /l.
Sources of error. Automated cell counters use a variety of technologies. They perform well for normal blood. There are limitations with some counters, for example nucleated red cells can be counted as lymphocytes and large immature platelets can be included in the erythrocyte count. Automated differential counts are reasonably accurate for normal cells but are less reliable when handling abnormal/immature cells. The automated differential count must always be supported byblood film examination. Haemocytometers, used for manual counts, have counting areas on both sides of the chamber. The number of cells counted from each side should vary by <10% for WBC and <20% for RBC. If variation is greater than this, the cell suspension is not evenly distributed and the counts should be repeated. Manual cell counts incur significant error, for example, ±20% may occur with WBCs. This should be taken into account when evaluating leucocyte changes.
Haemoglobin concentration. Haemoglobin (Hb) is included in the CBC on automated analysers. Conventionally in veterinary medicine the PCV is used to determine the adequacy of red cell mass, rather than Hb and the RBC count, since the microhaematocrit technique is a simple, accurate and inexpensive method. Haemoglobin may be more accurate if there is cell shrinkage, or swelling or increased cell fragility. Lipaemia can interfere with Hb concentration in photometric analysis. In feline blood, large numbers of Heinz bodies can also increase optical density causing artefactual increase in Hb.
Method for manual PCV. Microhaematocrit tubes are filled ⅔ - ¾ full with well mixed blood from the EDTA tube. This is centrifuged for 5 minutes at 11,500-15,000 rpm.
Schematic microhaematocrit tube***
Errors in the PCV are usually minimal but are most often related to centrifugation. Microhaematocrit tubes should be centrifuged for at least 5 minutes. If the PCV is >50%, red cells may pack incompletely, leading to overestimation of the PCV. Centrifuge speeds and brushes need to be checked regularly. Tubes should be evenly balanced in the centrifuge head to avoiduneven wear on the motor.
Mean Corpuscular Volume (MCV)
MCV (fl) = PCV (%) x 10 / Total RBC (x1012/l). This indicates the average size of RBCs.
Causes of decreased MCV (microcytosis):
- Artefact. Excess EDTA
- Iron deficiency. Maturation of the red cell and extrusion of the nucleus is stimulated by a critical concentration of haemoglobin. In iron deficiency, cell maturation is delayed allowing for one more cell division, leading to smaller cells
- Portosystemic shunts. Congenital or acquired through severe hepatic disease, probably due to abnormal iron metabolism
- Familial microcytosis. Occurs in the Akita and is of no clinical significances
Causes of increased MCV (macrocytosis):
- Regenerative anaemia. Reticulocytes are larger than mature erythrocytes
- Artefact. Sample ageing (may occur in samples submitted to external laboratories)
- Familial macrocytosis in poodles. Usually toy poodles. Any concurrent anaemia can be difficult to interpret as the increased MCV may suggest regeneration; a reticulocyte count should clarify. Hypersegmented neutrophils may also be present
- Autoagglutination. Spurious increase
- Myelodysplastic syndromes. May be associated with FeLV infection, concurrent non-regenerative anaemia
- Nutritional. Vitamin B12/folate deficiency
Mean Corpuscular Haemoglobin (MCH)
MCH (pg) = Hb (g/dl) x 10 / RBC (x 1012/l)
This indicates the mean weight of Hb per average RBC; it does not take account of the volume of the red cell.
Mean corpuscular Haemoglobin Concentration (MCHC)
MCHC (g/dl) = Hb (g/dl) x 100 / PCV (%)
This indicates the mean concentration of Hb per average RBC. It takes account of red cell volume and provides a more useful indication of the of amount of Hb present in red cells.
Red Cell Distribution Width (RDW)
This is available on automated haematology analysers using, a laser detection system in a flow cytometer, to measure the size and internal complexity of cells, based on light scatter at different angles. The RDW describes the variability in size of the RBCs and is a more sensitive indicator of altered red cell size then the MCV. RDW describes the entire red cell population and is increased with regenerative anaemia and iron deficiency anaemia.
Reticulocyte Count
Some automated in clinic analysers provide a reticulocyte count.
To perform a manual reticulocyte count, EDTA blood is mixed with an equal volume of 0.5% new methylene blue and left to stand for 10-20 minutes then remixed before preparing a blood smear.200-300 RBCs and reticulocytes are counted and the percentage of reticulocytes is calculated. The percentage may be reported but this can be misleading because the percentage is a ratio of reticulocytes to RBCs. In anaemia, the numbers of mature RBCs is reduced thus the severity exaggerates the percentage reticulocytes. The absolute reticulocyte count is more useful.
Absolute reticulocyte count (109/l) = percentage reticulocytes x RBC count (x 1012/l) x 10. An absolute reticulocyte count in the dog >60 x 109/l and in the cat >50 x 109/l is indicative of mildregeneration.
In cats, consideration of the numbers of both aggregate and punctate reticulocytes can help to determine the when anaemia began. Moderate to marked increases in aggregate reticulocytes with few punctate reticulocytes indicates recent anaemia (2-4 days). Increased punctate reticulocytes without increased aggregate reticulocytes indicates anaemia of 1-3 weeks duration or anaemia too mild to visualise an aggregate reticulocyte response.
Differential Cell Count
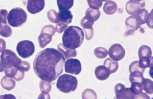
A manual differential cell count is obtained by identifying 100 nucleated cells on the smear. Automated haematology analysers may offer a 3 or 4 part differential count based on counting a much larger number of cells. The percentage of each cell type is multiplied by the total WBC count to provide absolute numbers of each. The automated differential count should always be validated by film examination and a manual differential count should be performed if any cell line falls significantly outside the reference interval. The differential count should include neutrophils, lymphocytes, monocytes, eosinophils, basophils and any immature or undifferentiated cells seen. Remember that the total WBC count should be corrected for the presence of NRBCs.
Platelet Count
These are recorded as number x109/l. Platelets are usually counted on a haematology analyser but can be counted manually with a haemocytometer. Numbers can be estimated from the blood smear. The average number of platelets is counted in 10 fields, using the x100 oil objective, in the monolayer of the smear. Normal dogs have 8-29 platelets per field and cats 10-29 per field. If a dog has a severe thrombocytopaenia only 1-2 platelets per field would be expected, approximating 15-30 x109/l. When numbers appear low, scan the smear to check platelet distribution is even and that there are no platelet clumps; these are usually found in the tail of the smear.
Examination of Blood Smear
The blood smear should not only be used for the differential count but to assess both numbers and morphology of RBCs, WBCs and platelets (remember to examine cells in the monolayer).
Erythrocyte evaluation
There are a number of terms used when examining RBCs, and numerous terms describing changes in red cell morphology. Only the most commonly encountered ones are described. Artefacts. Stain precipitate, refractile bodies and echinocytes. Echinocytes (crenated red cells). Red cells with many spiny projections which tend to be evenly spaced and uniform. This is often artefact seen in thick smears, which have taken a long time to dry, or due to excess EDTA, if the tube is not filled to the line. Echinocytes can be associated with pathological changes such as uraemia, glomerulonephritis, neoplasia and snake venom.
Indicators of regeneration
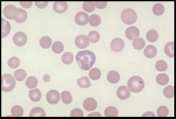
Anisocytosis. Refers to variation in RBC size. Immature cells (reticulocytes) are larger than mature erythrocytes.
Polychromasia. Describes the blue-grey colour of immature red cells, noted with Romanowsky stains. These immature cells are equivalent to reticulocytes in the dog and aggregate reticulocytes in cats. Grading them per high power field (hpf) helps to quantify the erythroid response particularly in dogs. They can be graded as shown below.
| Canine | Feline | |
|---|---|---|
| Occasional | <1 cell/hpf | <1 cell/hpf |
| 1+ | 2-4 cells/hpf | 1-2 cells/hpf |
| 2+ | 4-8 cells/hpf | 3-5 cells/hpf |
| 3+ | >8 cells/hpf | >5 cells/hpf |
The higher the grade, the more marked the degree of regeneration in response to anaemia. Anisocytosis and polychromasia in non-anaemic patients may reflect poor oxygenation of blood due to cardiac or respiratory disease.
Reticulocytes. Immature red cells, their residual RNA and mitochondria stain with new methylene blue.
Nucleated red blood cells (metarubricytes). These are immature red cells, which retain a condensed nucleus.
Immune mediated haemolytic anaemia (IMHA)
Agglutination. This describes the random, three dimensional clumping of red cells. Autoagglutination is persistent agglutination, which cannot be dispersed in saline. It is pathognomonic for IMHA but is not seen in all cases. Autoagglutination will affect red cell indices in automated analysers (increased MCV, MCH and MCHC).
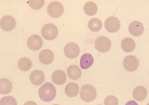
Spherocytes: These are spherical erythrocytes which appear smaller in diameter, stain more densely and lack central pallor. They are more obvious in canine blood (feline erythrocytes naturally lack central pallor) and are most commonly associated with IMHA. Other causes include snake bite, zinc toxicity and bee stings.
Other morphological changes
Acanthocytes. Erythrocytes with a few irregular, asymmetrical projections. In dogs acanthocytes may be associated with haemangiosarcoma, lymphoma, DIC and altered lipid metabolism (hepatic disease). In cats they may be recognised with liver disease including the cholangitis complex and hepatic lipidosis.
Basophilic stippling. Reflects the presence of aggregated ribosomal RNA in reticulocytes and is primarily associated with regenerative anaemias. It is also evident with the exaggerated regenerative response seen in many cases of lead poisoning (often in the absence of anaemia), due to the effect of lead on the bone marrow.
Eccentrocytes. These are RBCs where, as a result of oxidative injury, the Hb has coalesced, usually at one side of the cell; the remainder of the cell is pale staining.
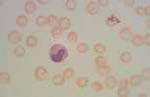
Heinz bodies. Oxidation of sulphydryl groups on the globin chains of haemoglobin results in the formation of Heinz bodies. These are refractile structures which occur along the internal surface of erythrocyte membranes, seen as pale pink-red projections with Romanowsky stains. Normal cats may have up to 5% Heinz bodies in their erythrocytes. Heinz body formation in the absence of anaemia may occur with feline diabetes mellitus, lymphoma and hyperthyroidism. Increased numbers of Heinz bodies, leading to haemolytic anaemia, may be associated with onion or garlic ingestion, kale or other brassica species consumption by ruminants and consumption of red maple leaves by alpacas and horses.
Howell-Jolly bodies. These are remnants of nuclear material . They are present in regenerative anaemias possibly due to inability of macrophages to fully remove the nuclei of maturing RBCs during accelerated production. If present without polychromasia, reduced macrophage function could be considered. They may be a common finding following splenectomy.
Hypochromasia. Pale erythrocytes with a reduced haemoglobin concentration (decreased MCHC). Seen in regenerative anaemia and with iron deficiency.
Red cell fragmentation. Small fragments are called schistocytes (schizocytes), larger red cell fragments include keratocytes (blister and helmet cells). These reflect metabolic disease or, more commonly, intravascular trauma associated with turbulent blood flow due to cardiac valvular disease, with passage through numerous capillaries in haemangiosarcoma and certain other malignant neoplasms, or fibrin strands cleaving erythrocytes in myelofibrosis or DIC. Severe hepatic disease may also result in red cell fragmentation.
Rouleaux. These are stacks of erythrocytes resembling a pile of coins, which disperse in saline. Rouleaux are most obvious when examining the body of the blood smear.
Rouleaux are prominent in blood smears from normal horses and some cats. Rouleaux are alsoseen in animals with hyperglobulinaemia, particularly h yperfibrinogenaemia and elevated acute phase proteins.
Target cells (codocytes). These are bell-shaped in-vivo but resemble a target on a blood smear due to accumulation of haemoglobin around the periphery and centre. They may be encountered with regenerative anaemia, renal, hepatic or lipid disorders.
Inclusions
Mycoplasma haemofelis (see image)
Babesia canis (see image)
Leucocyte evaluation
Normal blood should contain mature leucocyte populations which fall within reference limits for the species under consideration. A blood smear should be examined for abnormal cells prior to performing the differential cell count.
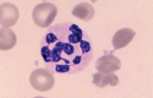
Neutrophil. A mature neutrophil has a segmented nucleus with condensed chromatin and fine intracytoplasmic azurophilic granules.
In females, the nucleus may have a drum-stick shaped appendage known as a Barr body which represents the inactive X chromosome; this is of no clinical significance.
Band neutrophil. This is an immature cell with an unsegmented nucleus showing parallel sides.The cytoplasm is similar to that of a mature cell. Sometimes less differentiated myeloid cells (metamyelocytes and myelocytes) are seen in the blood, usually associated with a severe acute inflammatory response. This is called a left shift.
Toxic neutrophil. Toxic change describes the leucocyte morphological abnormalities, seen predominantly in neutrophils, in the face of severe inflammation. The cytoplasm may show increased basophilia (light blue or grey rather than pink) and may contain Dohle bodies, which are small round or angular blue inclusions representing retained RNA (normally degraded during maturation). Dohle bodies are often seen in feline neutrophils and are not clinically significant unless frequent and prominent.
Neutrophils may have swollen nuclei which sometimes form doughnuts. Toxic change occurs in the bone marrow and reflects accelerated granulopoiesis, especiallyassociated with bacterial infections and IMHA.
There may be toxic granulation due to staining of primary granules. In severe toxaemia, neutrophils may show cytoplasmic vacuolation due to degranulation of lysosomes during disturbed maturation.
Hypersegmented neutrophil. There are more than 5 nuclear lobes. Found in dogs on corticosteroid therapy (which prolongs the half life of the neutrophil) and may be associated with uraemia. Also seen in Poodles with familial macrocytosis.
Distemper viral inclusions can occasionally be seen in neutrophils of infected dogs (see section on white cell inclusions).
Eosinophil. Mature eosinophils have a segmented nucleus and red intracytoplasmic granules. The granules in the dog are round and vary in size. In the cat they are small, uniform in size and rod shaped while in the horse the granules are round and very large. In rabbits, eosinophils have larger red granules than neutrophils although often the two cell types are difficult to differentiate and may be counted together as heterophils. Band eosinophils are immature cells showing less distinct nuclear segmentation.
Basophil. Rare in blood smears from healthy animals. Basophils are similar in size to eosinophils, with a segmented, ribbon-like nucleus and variable numbers of cytoplasmic granules. In dogs these are deep purple and sparse. Cats have more numerous lilac coloured granules with occasional deep purple granules.
Mast cell. These are not present in the blood of healthy animals but may be present with severe inflammatory disease or metastatic mast cell tumours (mastocytaemia). They are round cells with a round nucleus surrounded by a moderate volume of cytoplasm containing large numbers of purple granules.
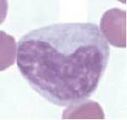
Lymphocyte. Mature lymphocytes are small cells with round, occasionally cleft nuclei which are approximately one red cell width in diameter; they have condensed nuclear chromatin and a narrow rim of pale cytoplasm. Reactive lymphocyte. Slightly larger than a mature lymphocyte. Cells have round nuclei with condensed nuclear chromatin, surrounded by a rim of pale basophilic cytoplasm.
Lymphoblast. A large cell with a large nucleus, two to three red cell widths in diameter, with one or more prominent nucleoli. These are rarely seen in blood smears from healthy animals but may be present with lymphoid neoplasia.
Large granular lymphocyte. Lymphocyte containing several prominent magenta coloured cytoplasmic granules. Rarely seen in peripheral blood but are numerous in large granular cell leukaemia in the dog
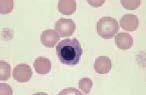
Monocyte. These are large cells with a deeply indented nucleus showing pale, finely granular nuclear chromatin. They have a moderate amount of blue-grey cytoplasm, which may contain vacuoles.
Leucocyte Inclusions
Canine distemper intracytoplasmic inclusion in a neutrophil (See image)
Ehrlichia canis morula in a monocyte (see image)
Leishmania in the cytoplasm of a neutrophil (see image)
Anaplasma platys elementary bodies (see image)
Hepatozoon gametocytes and Ehrlichia Morula (see image)
Platelet Evaluation
Platelet numbers can be estimated from the blood smear as described previously. The smear should be checked for any platelet clumps which are often found in the tail (feathered edge) of the smear. Clumping of platelets is frequently seen in cats and results in a spuriously low platelet count.
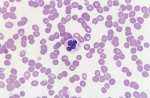
Macrothrombocyte. A large platelet indicating enhanced thrombopoiesis. Larger platelets are more functionally active and this may explain why some dogs with thrombocytopaenia, but largeplatelets, do not bleed.
Primary platelet disorders such as thrombasthenic thrombopathia of otter hounds are rare, producing large and morphologically bizarre platelets. Cavalier King Charles spaniels may have large platelets with decreased platelet counts due to inherited macrothrombocytopaenia.
Authors and References
NationWide Laboratories#In Partnership with NationWide Laboratories