Difference between revisions of "Leishmania"
Jump to navigation
Jump to search
Mariapavlou (talk | contribs) |
m |
||
| (67 intermediate revisions by 15 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{unfinished}} |
| − | + | ||
| − | {{ | + | {{toplink |
| − | | | + | |backcolour = |
| − | | | + | |linkpage =Parasites |
| − | | | + | |linktext =PARASITES |
| − | | | + | |pagetype=Bugs |
| − | | | + | |sublink1=Protozoa |
| − | | | + | |subtext1=PROTOZOA |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
}} | }} | ||
| − | == | + | <br> |
| − | [[Image: | + | |
| − | [[Image: | + | ==''Trypanosoma''== |
| − | [[Image: | + | [[Image:Trypanosoma.jpg|thumb|right|150px|''Trypanosoma cruzi'' - CDC/Dr. Myron G. Schultz]] |
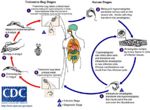
| − | '' | + | [[Image:T.cruzi Life cycle.jpg|thumb|right|150px|''T. cruzi'' Life Cycle Diagram - Wikimedia Commons]] |
| − | + | [[Image:Triatoma infestans.jpg|thumb|right|150px|''Triatoma infestans'' the Kissing bug - WHO Wikimedia Commons]] | |
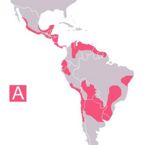
| − | + | [[Image:Chagas endemic zones 2005.jpg|thumb|right|150px|Chagas endemic zones 2005 - Wikimedia Commons]] | |
| − | + | *Protozoal parasites found in the blood and tissues of vertebrates | |
| + | |||
| + | *Worldwide distribution | ||
| + | |||
| + | *Causes sleeping sickness in humans | ||
| + | |||
| + | *Particularly affect sub-Saharan Africa | ||
| + | **Affect cattle production | ||
| + | **Cause Nagana (depression) | ||
| + | |||
| + | *Divided into two groups depending on the mode of development of the insect vector | ||
| + | **'''Salivarian''' | ||
| + | ***Multiply in the foregut | ||
| + | ***Transmitted via innoculation via feeding | ||
| + | **'''Stercorarian''' | ||
| + | ***Multiply in the hindgut | ||
| + | ***Transmitted via contamination of wounds with insect faeces | ||
| + | |||
| + | '''Life Cycle''' | ||
| + | *Undergo morphological transformations in intermediate host before becoming infective for the next host | ||
| + | |||
| + | *Blood-sucking [[Biting Flies|flies]] ingest trypanosomes whilst taking a blood meal from an infected animal | ||
| + | **Trypanosomes multiply first in the gut of the [[Biting Flies|fly]] | ||
| + | |||
| + | *Salivarian trypanosomes are transmitted by [[Biting Flies#Glossinidae|Tsetse flies]] | ||
| + | **Trypanosomes pass foward to the salivary glands where they transform into the infective stage | ||
| + | **Innoculated with saliva when [[Biting Flies#Glossinidae|Tsetse fly]] next feeds on a host | ||
| + | |||
| + | *Stercorarian trypanosomes are transmitted by triatomid bugs, [[Biting Flies#Tabanidae|tabanids]] and [[Biting Flies#Melophagus spp.|keds]] | ||
| + | **Trypanosomes pass back to the rectum | ||
| + | **Next host is infected when skin wounds are contaminated with infected [[Insecta|insect]] faeces | ||
| + | |||
| + | '''Pathogenesis''' | ||
| + | *Salivarian | ||
| + | **Causes wasting disease in cattle (nagana) | ||
| + | **Sleeping sickness in humans | ||
| + | |||
| + | *Stercorarian | ||
| + | **''T. cruzi'' most important in veterinary medicine | ||
| + | ***Occurs in South America | ||
| + | ***Infects armadillos, possums and humans | ||
| + | ***Causes Chagas Disease | ||
| + | **Transmitted by a triatomid (kissing) bug | ||
| + | **Chronic infections are often fatal causing heart failure | ||
| + | **Non-pathogenic species are transmitted by [[Biting Flies#Tabanidae|tabanids]] and [[Biting Flies#Melophagus spp.|keds]] | ||
| + | ***''T. theileria'' and ''T. melophagium'' | ||
| + | |||
| + | *Enlarged [[Lymph Nodes - Anatomy & Physiology|lymph nodes]] and [[Spleen - Anatomy & Physiology|spleen]] | ||
| + | **Causes lymphoid exhaustion | ||
| + | |||
| + | *Anaemia | ||
| + | **Red blood cells removed from circulation | ||
| + | |||
| + | *Degeneration and inflammation of multiple organs | ||
| + | **E.g. Skeletal muscle, myocardium and CNS | ||
| + | |||
| + | '''Epidemiology''' | ||
| + | *Vector distribution | ||
| + | **[[Biting Flies#Glossinidae|Tsetse flies]] found in riverine, savannah and forest habitats | ||
| + | **Up to 20% [[Biting Flies|flies]] infected | ||
| + | **[[Biting Flies|Flies]] infected for life | ||
| + | |||
| + | *Parasite virulence | ||
| + | **Some parasitaemic animals survive for long periods of time | ||
| + | ***E.g. ''T. brucei'' and ''T. congolense'' | ||
| + | ***Increases the opportunity for infection of [[Biting Flies|flies]] | ||
| + | **Some trypanosomes kill their host in 1-2 weeks | ||
| + | ***E.g. ''T. vivax'' | ||
| + | ***Decreases the chances of [[Biting Flies|fly]] infection | ||
| + | **Trypanosomes avoid host immune defences by altering glycoprotein coat (surface antigen) before host [[Immunoglobulins - WikiBlood|antibody]] response | ||
| + | ***'''Antigenic variation''' can occur many times over several months causes relapsing parasitaemia | ||
| + | |||
| + | *Host response | ||
| + | **Trypanotolerant wild animals remain parasitaemic for prolpnged periods without showing clincial signs of disease | ||
| + | ***Cause lasting reservoirs of infection | ||
| + | **Most domestic livestock are susceptible to trypanosomosis | ||
| + | **Some local breeds of sheep, goats and cattle are trypanotolerant | ||
| + | ***E.g. ''Bos indicus'' | ||
| − | + | '''Diagnosis''' | |
| − | '' | + | *Demonstrate trypanosomes in blood |
| + | **Giemsa stained smears | ||
| + | **Fresh blood films | ||
| + | ***Motile trypanosomes | ||
| + | **Haematocrit tube | ||
| + | ***Motile trypanosomes at the plasma/buffy coat interface | ||
| − | + | '''Control''' | |
| − | + | *[[Biting Flies#Glossinidae|Tsetse fly]] control | |
| + | **Spraying and trapping | ||
| − | + | *Prophylactic drug treatment | |
| + | **Change drug group periodically to decrease the chances of resistance occuring | ||
| + | **May lead to protective immunity but livestock will still be susceptible to heterologous challenges | ||
| − | + | *Barrier fences and buffer zones | |
| + | **Separate livestock and wild animals | ||
| − | + | *Trypanotolerant livestock | |
| − | |||
| − | + | '''Other trypanosomes''' | |
| + | *Mechanically transmitted by [[Biting Flies|biting flies]] | ||
| + | **E.g. Surra affecting horses and camels in North Africa, Asia and South America | ||
| + | **''T. equinum'' in South America | ||
| + | **''T. evansi'' in Asia | ||
| − | + | *Venerally transmitted | |
| − | + | **E.g. Dourine | |
| + | ***Transmitted by ''T. equiperdum'' | ||
| + | ***Causes genital and abdominal oedema, emaciataion and CNS signs | ||
| + | ***Affects horses and donkeys in Africa, Asia, Central and South America | ||
| − | + | *Non-pathogenic species occur in the UK | |
| + | **In sheep caused by ''T. melophagium'' | ||
| + | **In cattle caused by ''T. theileri'' | ||
| − | == | + | ==''Leishmania''== |
| − | |||
| − | + | *''Leishmania'' spp. are intracellular parasites of [[Macrophage|macrophages]] | |
| − | |||
| − | + | *Are closely related to ''Trypanosoma'' spp. | |
| − | |||
| − | + | *Cause diseases in humans, dogs and wild animals | |
| − | + | *Present in southern Europe, Africa, Asia and South America | |
| − | | | + | |
| − | | | + | '''Life Cycle''' |
| − | [[ | + | *Transmitted by blood sucking [[Biting Flies#Psychodidae|sand flies]] |
| − | | | + | **''Phlebotomus'' spp. in the Old World |
| − | + | **''Lutzomyia'' spp. in the New World | |
| + | |||
| + | *The amastigote (morphological form) in found in vertebrate [[Macrophage|macrophages]] | ||
| + | |||
| + | *Ingested by [[Biting Flies#Psychodidae|sand fly]] during feeding | ||
| + | **Transforms in [[Insecta|insect]] gut | ||
| + | |||
| + | *Multiplies and migrates to [[Insecta|insect]] proboscis | ||
| + | **Innoculated during feeding | ||
| + | **Can be transmitted percutaneously if [[Biting Flies#Psychodidae|sand fly]] crushed on skin | ||
| + | |||
| + | *Invades [[Macrophage|macrophages]] and reverts to amastigote | ||
| + | |||
| + | '''Pathogenesis''' | ||
| + | *Infection of vertebrate host | ||
| + | **Produces foci of proliferating ''Leishmania''-infected [[Macrophage|macrophages]] in skin ('''cutaneous''') or internal organs ('''visceral''') | ||
| + | |||
| + | *Very long incubation periods | ||
| + | **Months to years | ||
| + | |||
| + | *Many infected dogs are asymptomatic | ||
| + | |||
| + | *Cutaneous form | ||
| + | **Produces areas of ulceration on pinnae of ears | ||
| + | |||
| + | *Visceral form causes chronic wasting condition | ||
| + | **Generalised eczema | ||
| + | ***Loss of hair around eyes producing 'spectacle' effect | ||
| + | **Intermittent fever | ||
| + | **Generalised lymphadenopathy | ||
| + | |||
| + | *Involved in [[Parasitic skin infections - Pathology#Protozoa|skin infections]] | ||
| + | |||
| + | '''Epidemiology''' | ||
| + | *Disease dependent on [[Biting Flies#Psychodidae|sand fly]] vectors | ||
| + | **E.g. Common in dogs around the Mediterranean coast, foci around southern Europe and around Madrid | ||
| + | |||
| + | *Reservoirs of infection | ||
| + | **E.g. Wild animals such as rodents and stray dogs | ||
| + | |||
| + | *Mechanisms of transmission | ||
| + | **Direct contact | ||
| + | **[[Biting Flies#Psychodidae|sand fly]] bite | ||
| + | |||
| + | *Leishmaniasis in British dogs | ||
| + | **Susceptible to infection if exposed whilst abroad in endemic areas as have no immunity | ||
| + | **No [[Biting Flies#Psychodidae|sand flies]] in Britain but dogs have become infected whilst in contact with infected imported animals | ||
| + | '''Diagnosis''' | ||
| + | *Demonstrate ''Leishmania'' organisms | ||
| + | **In skin scraping or smears | ||
| + | **In [[Lymph Nodes - Anatomy & Physiology|lymph node]] or [[Bone Marrow - Anatomy & Physiology|bone marrow]] biopsies | ||
| − | + | '''Treatment and Control''' | |
| + | *Chemotherapy | ||
| + | **Prolonged treatment, expensive, suppresses infection | ||
| + | **Does not cure infection | ||
| − | + | *Prevent [[Biting Flies#Psychodidae|sand flies]] biting | |
| + | **Collars, sprays containing [[Ectoparasiticides|insecticide]] with repellent effect | ||
| − | + | *Destruction of infected and stray dogs | |
| − | [[ | + | **[[Biting Flies#Psychodidae|Sand flies]] biting infected dogs may spread the disease to to other dogs, humans and wildlife |
| − | + | **There is a slight possibility of transmission to humans by direct contact | |
Revision as of 17:16, 23 November 2008
| This article is still under construction. |
|
|
Trypanosoma
- Protozoal parasites found in the blood and tissues of vertebrates
- Worldwide distribution
- Causes sleeping sickness in humans
- Particularly affect sub-Saharan Africa
- Affect cattle production
- Cause Nagana (depression)
- Divided into two groups depending on the mode of development of the insect vector
- Salivarian
- Multiply in the foregut
- Transmitted via innoculation via feeding
- Stercorarian
- Multiply in the hindgut
- Transmitted via contamination of wounds with insect faeces
- Salivarian
Life Cycle
- Undergo morphological transformations in intermediate host before becoming infective for the next host
- Blood-sucking flies ingest trypanosomes whilst taking a blood meal from an infected animal
- Trypanosomes multiply first in the gut of the fly
- Salivarian trypanosomes are transmitted by Tsetse flies
- Trypanosomes pass foward to the salivary glands where they transform into the infective stage
- Innoculated with saliva when Tsetse fly next feeds on a host
- Stercorarian trypanosomes are transmitted by triatomid bugs, tabanids and keds
- Trypanosomes pass back to the rectum
- Next host is infected when skin wounds are contaminated with infected insect faeces
Pathogenesis
- Salivarian
- Causes wasting disease in cattle (nagana)
- Sleeping sickness in humans
- Stercorarian
- T. cruzi most important in veterinary medicine
- Occurs in South America
- Infects armadillos, possums and humans
- Causes Chagas Disease
- Transmitted by a triatomid (kissing) bug
- Chronic infections are often fatal causing heart failure
- Non-pathogenic species are transmitted by tabanids and keds
- T. theileria and T. melophagium
- T. cruzi most important in veterinary medicine
- Enlarged lymph nodes and spleen
- Causes lymphoid exhaustion
- Anaemia
- Red blood cells removed from circulation
- Degeneration and inflammation of multiple organs
- E.g. Skeletal muscle, myocardium and CNS
Epidemiology
- Vector distribution
- Tsetse flies found in riverine, savannah and forest habitats
- Up to 20% flies infected
- Flies infected for life
- Parasite virulence
- Some parasitaemic animals survive for long periods of time
- E.g. T. brucei and T. congolense
- Increases the opportunity for infection of flies
- Some trypanosomes kill their host in 1-2 weeks
- E.g. T. vivax
- Decreases the chances of fly infection
- Trypanosomes avoid host immune defences by altering glycoprotein coat (surface antigen) before host antibody response
- Antigenic variation can occur many times over several months causes relapsing parasitaemia
- Some parasitaemic animals survive for long periods of time
- Host response
- Trypanotolerant wild animals remain parasitaemic for prolpnged periods without showing clincial signs of disease
- Cause lasting reservoirs of infection
- Most domestic livestock are susceptible to trypanosomosis
- Some local breeds of sheep, goats and cattle are trypanotolerant
- E.g. Bos indicus
- Trypanotolerant wild animals remain parasitaemic for prolpnged periods without showing clincial signs of disease
Diagnosis
- Demonstrate trypanosomes in blood
- Giemsa stained smears
- Fresh blood films
- Motile trypanosomes
- Haematocrit tube
- Motile trypanosomes at the plasma/buffy coat interface
Control
- Tsetse fly control
- Spraying and trapping
- Prophylactic drug treatment
- Change drug group periodically to decrease the chances of resistance occuring
- May lead to protective immunity but livestock will still be susceptible to heterologous challenges
- Barrier fences and buffer zones
- Separate livestock and wild animals
- Trypanotolerant livestock
Other trypanosomes
- Mechanically transmitted by biting flies
- E.g. Surra affecting horses and camels in North Africa, Asia and South America
- T. equinum in South America
- T. evansi in Asia
- Venerally transmitted
- E.g. Dourine
- Transmitted by T. equiperdum
- Causes genital and abdominal oedema, emaciataion and CNS signs
- Affects horses and donkeys in Africa, Asia, Central and South America
- E.g. Dourine
- Non-pathogenic species occur in the UK
- In sheep caused by T. melophagium
- In cattle caused by T. theileri
Leishmania
- Leishmania spp. are intracellular parasites of macrophages
- Are closely related to Trypanosoma spp.
- Cause diseases in humans, dogs and wild animals
- Present in southern Europe, Africa, Asia and South America
Life Cycle
- Transmitted by blood sucking sand flies
- Phlebotomus spp. in the Old World
- Lutzomyia spp. in the New World
- The amastigote (morphological form) in found in vertebrate macrophages
- Multiplies and migrates to insect proboscis
- Innoculated during feeding
- Can be transmitted percutaneously if sand fly crushed on skin
- Invades macrophages and reverts to amastigote
Pathogenesis
- Infection of vertebrate host
- Produces foci of proliferating Leishmania-infected macrophages in skin (cutaneous) or internal organs (visceral)
- Very long incubation periods
- Months to years
- Many infected dogs are asymptomatic
- Cutaneous form
- Produces areas of ulceration on pinnae of ears
- Visceral form causes chronic wasting condition
- Generalised eczema
- Loss of hair around eyes producing 'spectacle' effect
- Intermittent fever
- Generalised lymphadenopathy
- Generalised eczema
- Involved in skin infections
Epidemiology
- Disease dependent on sand fly vectors
- E.g. Common in dogs around the Mediterranean coast, foci around southern Europe and around Madrid
- Reservoirs of infection
- E.g. Wild animals such as rodents and stray dogs
- Mechanisms of transmission
- Direct contact
- sand fly bite
- Leishmaniasis in British dogs
- Susceptible to infection if exposed whilst abroad in endemic areas as have no immunity
- No sand flies in Britain but dogs have become infected whilst in contact with infected imported animals
Diagnosis
- Demonstrate Leishmania organisms
- In skin scraping or smears
- In lymph node or bone marrow biopsies
Treatment and Control
- Chemotherapy
- Prolonged treatment, expensive, suppresses infection
- Does not cure infection
- Prevent sand flies biting
- Collars, sprays containing insecticide with repellent effect
- Destruction of infected and stray dogs
- Sand flies biting infected dogs may spread the disease to to other dogs, humans and wildlife
- There is a slight possibility of transmission to humans by direct contact