Difference between revisions of "Lizard and Snake Specimen Collection and Evaluation"
| (18 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| + | {{unfinished}} | ||
| + | Diagnostic aids that are used routinely with more familiar domestic animals are also applicable to lizards and snakes. Those commonly available in veterinary practice include faecal examination, microbiology, cytology, [[Lizard and Snake Haematology|haematology]] and [[Lizard and Snake Biochemistry|biochemistry]], [[Lizard and Snake Imaging|radiography]] and biopsy. This section will cover specimen collection and interpretation. | ||
[[Image:Culture_copy.jpg|250px|thumb|right|(Copyright © RVC)]] | [[Image:Culture_copy.jpg|250px|thumb|right|(Copyright © RVC)]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
==Colonic wash== | ==Colonic wash== | ||
| − | A lubricated catheter with an attached syringe is gently inserted through the vent. The catheter should pass easily into the colon and if resistance is met it is pushing against the dorsal wall of the cloaca. Up to approximately 10ml/kg of sterile saline is injected into the colon. The fluid is massaged prior to aspiration. | + | A lubricated catheter with an attached syringe is gently inserted through the vent. The catheter should pass easily into the colon and if resistance is met it is pushing against the dorsal wall of the cloaca. Up to approximately 10ml/kg of sterile saline is injected into the colon. The fluid is massaged prior to aspiration. Learn about digestion in [[Lizard Gastrointestinal System|lizards]] and [[Snake Digestive System|snakes]] |
| − | |||
==Faecal evaluation== | ==Faecal evaluation== | ||
Evaluation of the faeces for protozoa, amoeba and nematodes is considered part of a minimum database for lizards. Most reptiles defecate infrequently, especially if they have been ill and anorexic. Faeces can be obtained by i) collection of normally voided faeces, ii) bathing to stimulate defecation and iii) colonic wash - the rectum is quite distensible. A lubricated large gauge tube is inserted into the colon through the vent and approximately 10 ml/kg of sterile water is injected into the colon. The fluid is massaged prior to aspiration. | Evaluation of the faeces for protozoa, amoeba and nematodes is considered part of a minimum database for lizards. Most reptiles defecate infrequently, especially if they have been ill and anorexic. Faeces can be obtained by i) collection of normally voided faeces, ii) bathing to stimulate defecation and iii) colonic wash - the rectum is quite distensible. A lubricated large gauge tube is inserted into the colon through the vent and approximately 10 ml/kg of sterile water is injected into the colon. The fluid is massaged prior to aspiration. | ||
| Line 13: | Line 10: | ||
===Wet mount=== | ===Wet mount=== | ||
Make a direct wet mount and examine under low power for nematode larvae and high power for protozoa. | Make a direct wet mount and examine under low power for nematode larvae and high power for protozoa. | ||
| − | *Water preparation - nematode ova and moving flagellates (concentrated saline may destroy flagellates immediately) | + | *Water preparation - nematode ova and moving flagellates (concentrated saline may destroy flagellates immediately) iodine stain (e.g. Lugol's) or eosin - identification of encysted entamoeba. |
| − | + | *Gram stain - Though they are frequently recovered from clinically healthy captive reptiles, most pathogenic infections are caused by Gram negative pathogens e.g. Pseudomonas, Aeromonas, Proteus, Salmonella. | |
| − | *Gram stain - Though they are frequently recovered from clinically healthy captive reptiles, most pathogenic infections are caused by Gram negative pathogens e.g. | ||
| − | |||
===Flotation=== | ===Flotation=== | ||
The faecal material is mixed with the high specific gravity flotation fluid. The debris is removed with a filter and material that is less dense than the flotation fluid, such as nematode ova, will rise to the top. This can then be examined on a microscope slide. | The faecal material is mixed with the high specific gravity flotation fluid. The debris is removed with a filter and material that is less dense than the flotation fluid, such as nematode ova, will rise to the top. This can then be examined on a microscope slide. | ||
| Line 34: | Line 29: | ||
*''Tissue for virus isolation'' - For viral isolation, place tissue sections in individual sterile bags and freeze. | *''Tissue for virus isolation'' - For viral isolation, place tissue sections in individual sterile bags and freeze. | ||
===Bone marrow from snakes=== | ===Bone marrow from snakes=== | ||
| − | Bone marrow from snakes is difficult but not impossible to obtain. The bone marrow cavity of the ribs of large snakes will yield a sample. The area around the dorsal fourth of a rib is aseptically prepared. The skin is incised and the cortical bone of the rib is drilled through with a 21 to 23 gauge spinal needle. The needle is then advanced a few millimetres to the marrow cavity. For smaller species, surgical removal of a portion of a rib or vertebral spinous process is possible. It is fixed in formal saline and placed in a decalcifying solution until soft enough to allow histological processing. It is also possible to obtain marrow from the ventral vertebral bodies by biopsy. | + | Bone marrow from snakes is difficult but not impossible to obtain from snakes. The bone marrow cavity of the ribs of large snakes will yield a sample. The area around the dorsal fourth of a rib is aseptically prepared. The skin is incised and the cortical bone of the rib is drilled through with a 21- to 23-gauge spinal needle. The needle is then advanced a few millimetres to the marrow cavity. For smaller species, surgical removal of a portion of a rib or vertebral spinous process is possible. It is fixed in formal saline and placed in a decalcifying solution until soft enough to allow histological processing. It is also possible to obtain marrow from the ventral vertebral bodies by biopsy. |
| − | |||
==Swab== | ==Swab== | ||
| − | Culture sites should be vigorously sampled. | + | Culture sites should be vigorously sampled. Culture blood if septicaemia is suspected. Swabs for bacteriology should immediately go into transport media if there is any delay in culturing. It is preferable to incubate at 25°C and 37°C. It is advisable to take a second swab and roll this onto a microscope slide for gram staining. Culturing of fluid within the oral cavity usually gives non-diagnostic results. Preferable methods, as appropriate, are biopsy of oral lesions, sampling high within the choanal slit or tracheal sampling (by passing a microswab through the glottis). |
| − | |||
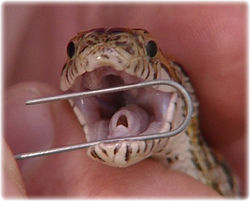
==Transtracheal wash== | ==Transtracheal wash== | ||
Respiratory disease is common in reptiles and sample collection from the respiratory tract may be essential for diagnosis and treatment. Transtracheal washes should therefore be a routine procedure for all lizards and snakes with respiratory disease. They may be collected in physically restrained snakes but chemical restraint is usually required. | Respiratory disease is common in reptiles and sample collection from the respiratory tract may be essential for diagnosis and treatment. Transtracheal washes should therefore be a routine procedure for all lizards and snakes with respiratory disease. They may be collected in physically restrained snakes but chemical restraint is usually required. | ||
| − | [[Image:Glottis.jpg|250px|thumb|right| | + | [[Image:Glottis.jpg|250px|thumb|right|Copyright © RVC)]] |
*Collection | *Collection | ||
The patient is positioned with the head slightly elevated. Identify the position of the heart. The jaws are opened and the glottis identified. Upon inspiration a sterile catheter is introduced to the appropriate level. The lung field is caudal to the heart in boids and colubrids. Sterile saline equal to 0.5-1% bodyweight is injected. The head is then lowered and the fluid aspirated. Occasionally more is aspirated than injected and this usually indicates respiratory disease. Any fluid left in the lungs is absorbed. Use of endoscopy allows visualisation as well as sampling. Samples of low cellularity can be concentrated using centrifugation. | The patient is positioned with the head slightly elevated. Identify the position of the heart. The jaws are opened and the glottis identified. Upon inspiration a sterile catheter is introduced to the appropriate level. The lung field is caudal to the heart in boids and colubrids. Sterile saline equal to 0.5-1% bodyweight is injected. The head is then lowered and the fluid aspirated. Occasionally more is aspirated than injected and this usually indicates respiratory disease. Any fluid left in the lungs is absorbed. Use of endoscopy allows visualisation as well as sampling. Samples of low cellularity can be concentrated using centrifugation. | ||
| Line 54: | Line 47: | ||
==Blood collection== | ==Blood collection== | ||
Find out more about collecting blood from [[Lizard Blood Collection|lizards]] and [[Snake Blood Collection|snakes]]. | Find out more about collecting blood from [[Lizard Blood Collection|lizards]] and [[Snake Blood Collection|snakes]]. | ||
| − | + | *Check up on giving physical examinations on [[Lizard Physical Examination|lizards]] and [[Snake Physical Examination|snakes]]. | |
| − | + | [[Category:Lizard and Snake Diagnostics]] | |
| − | | | + | [[Category:Lizard_Specimen_Collection]] |
| − | + | [[Category:Lizard_Specimen_Collection]] | |
| − | [ | + | [[Category:Snake_Specimen_Collection]] |
| − | |||
| − | [ | ||
| − | |||
| − | [ | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | [[Category: | ||
| − | [[Category: | ||
Revision as of 21:05, 6 May 2010
| This article is still under construction. |
Diagnostic aids that are used routinely with more familiar domestic animals are also applicable to lizards and snakes. Those commonly available in veterinary practice include faecal examination, microbiology, cytology, haematology and biochemistry, radiography and biopsy. This section will cover specimen collection and interpretation.
Colonic wash
A lubricated catheter with an attached syringe is gently inserted through the vent. The catheter should pass easily into the colon and if resistance is met it is pushing against the dorsal wall of the cloaca. Up to approximately 10ml/kg of sterile saline is injected into the colon. The fluid is massaged prior to aspiration. Learn about digestion in lizards and snakes
Faecal evaluation
Evaluation of the faeces for protozoa, amoeba and nematodes is considered part of a minimum database for lizards. Most reptiles defecate infrequently, especially if they have been ill and anorexic. Faeces can be obtained by i) collection of normally voided faeces, ii) bathing to stimulate defecation and iii) colonic wash - the rectum is quite distensible. A lubricated large gauge tube is inserted into the colon through the vent and approximately 10 ml/kg of sterile water is injected into the colon. The fluid is massaged prior to aspiration.
Sedimentation
The sediment can be examined after a faecal sample is centrifuged.
Wet mount
Make a direct wet mount and examine under low power for nematode larvae and high power for protozoa.
- Water preparation - nematode ova and moving flagellates (concentrated saline may destroy flagellates immediately) iodine stain (e.g. Lugol's) or eosin - identification of encysted entamoeba.
- Gram stain - Though they are frequently recovered from clinically healthy captive reptiles, most pathogenic infections are caused by Gram negative pathogens e.g. Pseudomonas, Aeromonas, Proteus, Salmonella.
Flotation
The faecal material is mixed with the high specific gravity flotation fluid. The debris is removed with a filter and material that is less dense than the flotation fluid, such as nematode ova, will rise to the top. This can then be examined on a microscope slide.
Acid fast
Acid fast stains can be used to identify Cryptosporidium.
Urinalysis
Urinalysis is not a useful diagnostic aid in reptiles since contamination and modification of the urine occurs in the cloaca and colon.
Urate examination
It is worthwhile examining the urates for unusual pigmentation. Urates stained with bile pigments have been associated with liver disease. Green-stained urates can be mixed with saline and coverslipped for microscopic examination. This may reveal amoebic cysts or operculated fluke ova.
Biopsy
The collection of multiple biopsies is often essential for diagnosis. Samples taken should include both normal and abnormal tissue. The same procedure is used for collection of tissues from necropsies. Skin biopsies can be collected relatively easily with physical and/or chemical restraint plus local anaesthetic. Single scales can be sampled since they contain dermis and epidermis. General anaesthesia will be necessary for subcutaneous masses. Intracoelomic samples can be collected by endoscopy or coeliotomy.
- Tissue for histology - Immediately after collection samples are placed in formal saline at a ratio of 1:10. After 48 hours the samples are then stored in 70% ethanol (storage in formalin modifies viral lesions).
- Tissue containing urates - Tissues containing suspected urate deposits should be fixed in methanol rather than formal saline because the methanol will preserve urate microcrystals, whereas formalin will dissolve them.
- Tissue for electron microscopy - Tissues submitted for electron microscopy must be as fresh as possible and cut into 1.0 mm blocks and placed immediately into glutaraldehyde solution.
- Tissue containing bone - Tissues containing bone should be formalin-fixed then treated with a decalcifying solution.
- Tissue for virus isolation - For viral isolation, place tissue sections in individual sterile bags and freeze.
Bone marrow from snakes
Bone marrow from snakes is difficult but not impossible to obtain from snakes. The bone marrow cavity of the ribs of large snakes will yield a sample. The area around the dorsal fourth of a rib is aseptically prepared. The skin is incised and the cortical bone of the rib is drilled through with a 21- to 23-gauge spinal needle. The needle is then advanced a few millimetres to the marrow cavity. For smaller species, surgical removal of a portion of a rib or vertebral spinous process is possible. It is fixed in formal saline and placed in a decalcifying solution until soft enough to allow histological processing. It is also possible to obtain marrow from the ventral vertebral bodies by biopsy.
Swab
Culture sites should be vigorously sampled. Culture blood if septicaemia is suspected. Swabs for bacteriology should immediately go into transport media if there is any delay in culturing. It is preferable to incubate at 25°C and 37°C. It is advisable to take a second swab and roll this onto a microscope slide for gram staining. Culturing of fluid within the oral cavity usually gives non-diagnostic results. Preferable methods, as appropriate, are biopsy of oral lesions, sampling high within the choanal slit or tracheal sampling (by passing a microswab through the glottis).
Transtracheal wash
Respiratory disease is common in reptiles and sample collection from the respiratory tract may be essential for diagnosis and treatment. Transtracheal washes should therefore be a routine procedure for all lizards and snakes with respiratory disease. They may be collected in physically restrained snakes but chemical restraint is usually required.
- Collection
The patient is positioned with the head slightly elevated. Identify the position of the heart. The jaws are opened and the glottis identified. Upon inspiration a sterile catheter is introduced to the appropriate level. The lung field is caudal to the heart in boids and colubrids. Sterile saline equal to 0.5-1% bodyweight is injected. The head is then lowered and the fluid aspirated. Occasionally more is aspirated than injected and this usually indicates respiratory disease. Any fluid left in the lungs is absorbed. Use of endoscopy allows visualisation as well as sampling. Samples of low cellularity can be concentrated using centrifugation.
- Evaluation - The sample can be used for:
- A wet mount for microscopy for parasite
- Cytology
- Bacteriology (including both aerobic and anaerobic culture)
- Other tests as necessary including fungal culture and virology
Gastric lavage
Stomach washings can be obtained for preparation of wet mounts, cytology and microbiology. A lubricated catheter is introduced into the stomach via the oesophagus and sterile saline is injected. The stomach is massaged and the fluid is then withdrawn. The specimen can be concentrated by centifuging. Gastric lavage is commonly used in the diagnosis of cryptosporidiosis.
Blood collection
Find out more about collecting blood from lizards and snakes.