Difference between revisions of "Trypanosomosis - Donkey"
| Line 52: | Line 52: | ||
* If quinpyramine is used, donkeys should be well-rested beforehand, as heat, stress, and fatigue can precipitate acute curare-like reactions after treatment | * If quinpyramine is used, donkeys should be well-rested beforehand, as heat, stress, and fatigue can precipitate acute curare-like reactions after treatment | ||
* <u>Once nervous signs occur, treatment for trypanosomosis is unlikely to be successful</u>, as trypanocides do not cross the blood-brain barrier | * <u>Once nervous signs occur, treatment for trypanosomosis is unlikely to be successful</u>, as trypanocides do not cross the blood-brain barrier | ||
| − | * [[Donkey | + | * [[Euthanasia - Donkey|Euthanasia]] is often recommended for dourine where there is an ongoing eradication programme, as treatment may improve clinical signs but will not sterilise the infection and the animal will remain as a carrier. A more widely applicable strategy for dourine is a combination of treatment and castration |
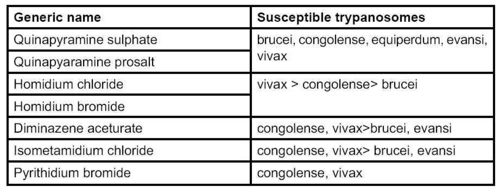
[[Image:Trypanosomosis drugs donkey.jpg|center|thumb|500px|<small><center>Drugs that have been used to treat equine trypanosomosis. (Image courtesy of [http://drupal.thedonkeysanctuary.org.uk The Donkey Sanctuary])</center></small>]] | [[Image:Trypanosomosis drugs donkey.jpg|center|thumb|500px|<small><center>Drugs that have been used to treat equine trypanosomosis. (Image courtesy of [http://drupal.thedonkeysanctuary.org.uk The Donkey Sanctuary])</center></small>]] | ||
Revision as of 13:21, 23 February 2010
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
Introduction
Trypanosomosis in equines is caused by trypanosome protozoans, of which there are five pathogenic to livestock. Most common are:
- Trypasnosoma equiperdum, the cause of dourine, which is transmitted venereally
- T. evansi the cause of surra, which is transmitted by several species of tabanid flies
As with many other domestic livestock, tsetse-transmitted T. vivax, T. congolense and T. brucei can also affect equines in tsetse-infested areas. T. vivax can also be transmitted mechanically by biting flies, which extends its occurrence outside the tsetse belt.
Traditionally donkeys, but not horses, are found in some tsetse-infested areas, and horses can only be maintained in these areas with the use of trypanocidal drugs. The greater resistance of donkeys to tsetse-transmitted trypanosomosis is variable and may be due to donkeys being less attractive to these flies, having partial immunity to trypanosomes or other factors (Barrowman, 1991; Faye et al, 2001).
Tsetse-transmitted trypanosomosis occurs only in sub-Saharan Africa, affecting an area of 10,000,000 square kilometres. T. evansi is found in China, India, South-east Asia, North Africa, the Middle East, South America, Bulgaria and parts of the former USSR. T. equiperdum is reported in many countries in Asia, Africa, South America and south-easten Europe. In endemic areas, sero-prevalences are typically around 10%, but many of the affected equines will be asymptomatic. Mechanically transmitted T. vivax is present in South America.
Clinical features

Infection with tsetse-transmitted trypanosomosis can be acute or chronic. Signs are variable and dependent on the strain of trypanosomes, the general health status, level of stress, work, pregnancy status and any concurrent diseases. Enlarged lymph nodes, corneal opacity and petechial haemorrhages of the mucous membranes are also commonly reported signs with trypanosomosis. T. brucei is reported as causing serious acute disease in donkeys, whereas T. congolense can cause chronic wasting disease, leading to anaemia and oedema. T. vivax usually leads to chronic and milder disease. Mixed infections are also possible, as reported in Zimbabwe, where a high proportion of donkeys were found to harbour T. brucei and T. congolense, a combination which would be considered fatal in the horse (Boyt et al, 1972).
Donkeys infected with T. evansi are more resistant to clinical signs than horses but may show the signs mentioned above. Paralysis of the hind legs may occur in chronic cases. Mules are said to be as susceptible as horses to T. evansi (Uilenberg, 1998) but other texts indicate that a milder chronic form of the disease tends to occur in the mule (Luckins, 1999).
Infections with T. equiperdum often produce no or variable signs in the donkey, especially when caused by African strains. In general, infections are mild to asymptomatic, oedema in the genitals is not obvious and skin plaques occur in only 10% of infected cases. However, anaemia is often present (Kaufmann, 1996). In the horse, classic signs for dourine have been divided into three stages:
- One to two weeks after infection, genitals become swollen and there is vaginal discharge and patchy pigment loss in the mucosa of the vulva or penis.
- One month later, there are urticaria eruptions with plaques on the neck, chest flanks and rump.
- Progressive anaemia and limb paralysis may then follow, leading to death (Uilenberg, 1998; Connor, 2004).
Skin
Trypanosomiasis can present with persistent non-healing circular ulcers. A direct smear of the squeezed exudate from the lesion usually reveals large numbers of the parasites (usually T. brucei).
Diagnosis
- Diagnostic tests are derived from those developed and well documented in cattle (OIE, 2004)
- Blood samples may be taken from the peripheral ear vein and either examined as a wet smear or as a stained thin/thick smear. But, because levels of parasitemia fluctuate and in chronic cases can be low, it is often necessary to use a concentration method such as centrifugation and then look for trypanosomes in the blood buffy coat layer. Even this method
may detect as little as one third of cases
- Serology tests such as CFT and ELISA have been used
- Animal inoculation is not recommended as a diagnostic method on ethical grounds and in any case is not practicable in the field
- T. equiperdum can be isolated from genital discharge or urticarial plaques. However, as many cases are asymptomatic, serology is the most reliable and practicable test. CFT has been the standard test used for T. equiperdum, but should not be used where T. brucei spp. are present as crossreactions occur. In approximately 50% of cases donkey and mule sera show anti-complementarity. This can be reduced by dilution of the serum by half and heat inactivation at 60-63ºC for thirty minutes (OIE, 2004)
Treatment
- Treatment for equine trypanosomosis is extrapolated from regimes in other livestock. There is little empirical evidence for many trypanocides that are used in donkeys
- Trypanocidal drugs can be used for chemo-therapy/prophylaxis
- Resistance has been documented in donkeys (Assefa, 2001)
- The drugs shown in Table 2 (page 281) have been suggested for equines. They should be used with great caution, preferably after determining the trypanosome chemosensitivity and as part of integral approach to the disease
- Deep muscle injection divided through the day is recommended for some drugs, e.g. quinapyramine, because of tissue necrosis and toxicity. Adverse reactions are common with many trypanocides
- Diminazene at therapeutic doses can produce severe nervous signs and sometimes death in donkeys. It has been used as a preventative at low dosage every three months. Its success with mules for surra has been documented, as has the mild to severe toxicity observed when it was used (Tuntasuvan, 2003)
- Isometamidium chloride may be used for prevention and treatment, but careful aseptic precautions and deep intra-muscular injections are essential to prevent local tissue necrosis. Separate needles should be used for filling the syringe and giving the injection. A long, small-gauge needle should be used and firm pressure applied after withdrawal of the needle. Splitting the dose and giving in two sites is also recommended
- If quinpyramine is used, donkeys should be well-rested beforehand, as heat, stress, and fatigue can precipitate acute curare-like reactions after treatment
- Once nervous signs occur, treatment for trypanosomosis is unlikely to be successful, as trypanocides do not cross the blood-brain barrier
- Euthanasia is often recommended for dourine where there is an ongoing eradication programme, as treatment may improve clinical signs but will not sterilise the infection and the animal will remain as a carrier. A more widely applicable strategy for dourine is a combination of treatment and castration
Prevention
Some trypanocidal drugs can be used prophylactically but generally this is not common practice in donkeys. Traditional treatments are widely used in Africa and there is evidence that at least some of these products have some trypanocidal activity in vitro. However, as toxicity data is lacking, they should be used with caution, and on the basis of advice from local experts. Vector control can be used for controlling T. evansi and include:
- Stabling at night with fly-proof nets
- Avoid grazing near habitat such as woods, marshy grounds or river bank
- Control compost and manure which allow breeding of tabanids
- Use of insecticides and fly repellents
Extensive research has looked at the control of tsetse fly in livestock (Uilenberg, 1998). Community vector control is effective but is often not sustainable without external support. The individual donkey keeper can reduce risk and severity of infection by:
- Ensuring good nutrition
- Treating inter-current diseases
- Stabling donkeys at dawn and dusk
- Watering donkeys only at wells and pumps, and not at river watering places in West Africa, where riverine tetse are common
- Care should be taken to avoid iatrogenic transmission of T. vivax through needles or surgical equipment.
Dourine has been successfully eradicated from many areas by testing and slaughter. Where this is not feasible, the presence of fences and prevention of indiscriminate breeding may control spread of the disease. If possible, breeding animals should be tested serologically as the sperm and vaginal discharge of T. equiperdum infected donkeys is very virulent and can be a source of infection to other donkeys and horses.
References
- Anzuino, J. (2008) Exotic infections In Svendsen, E.D., Duncan, J. and Hadrill, D. (2008) The Professional Handbook of the Donkey, 4th edition, Whittet Books, Chapter 14
- Knottenbelt, D. (2008) Skin disorders In Svendsen, E.D., Duncan, J. and Hadrill, D. (2008) The Professional Handbook of the Donkey, 4th edition, Whittet Books, Chapter 8
|
|