| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
Description
Lymphangiectasia is a disease of the lymphatic vessels that results in the leakage of protein-rich lymph. The term is usually taken to mean intestinal lymphangiectasia (in which lymph is lost into the intestinal lumen, producing a protein-losing enteropathy(PLE) and severe lipid malabsorption) but thoracic and generalised lymphangiectasia have been reported.
Lymphangiectasia can be classified into a primary or secondary disease. Primary lymphangiectasia usually only affects the intestine but it occasionally involves a concurrent chylothorax. It occurs due to a congenital defect of the lymphatic vessels but it may be associated with inflammation of the lymphatics, so-called lipogranulomatous lympangitis. The relationship between lymphangiectasia and lipogranulomatous lymphangitis is currently unclear and it is possible that either condition could result in the development of the other. Secondary lymphangiectasia occurs with any pathological process that causes lymphatic obstruction, of which the most common are:
- Direct damage to the lymphatics
- Inflammation and subsequent fibrosis of the lymphatics, obstructing the lumina of the vessels.
- Neoplastic infiltration or erosion of the walls of lymphatic vessels.
- Obstruction of the thoracic duct, the major lymphatic vessel that runs through the chest. This may occur due to traumatic rupture or due to the presence of a neoplastic mass.
- Increased pressure in the systemic veins reducing the pressure gradient from the thoracic duct to the subclavian veins
- Right-sided heat failure due to cardiomyopathy, cardiac tamponade or tricuspid dysplasia, Cor Pulmonale or Cor Triatriatum Dexter.
- Obstruction to venous return by intra-thoracic masses including thymoma and thymic lymphoma.
Signalment
The disease is relatively common in dogs but rare in cats. Yorkshire terriers, Rottweilers and Norwegian Lundehunds are predisposed to the development of disease.
Diagnosis
Clinical Signs
Clinical signs are related to the loss of lymph and the resultant protein-losing enteropathy and fat malabsorption. The following signs are therefore common:
- Weight loss in the face of polyphagia due to loss of fat and protein.
- Chronic diarrhoea or steatorrhoea, the latter occurring due to the high fat content of the faeces. The presence of large quantities of fat in the intestinal lumen provides a substrate for bacteria which produce hydroxy-fatty acids as by-products. Bacterial proliferation may result in concurrent small intestinal bacterial overgrowth (SIBO) and the hydroxy-fatty acids act as potent secretagogues in the colon, leading to the production of diarrhoeic faeces.
- Effusions may develop for a number of reasons in animals with lymphangiectasia. Ascites composed of a transudate may develop in severely hypoproteinaemic animals but, in animals that develop secondary lymphangiectasia due to right-sided heart failure, a modified transudate may form due to portal hypertension. If the major lymphatic vessels of the abdomen are disrupted (by a neoplastic mass), chylous ascites may develop, although this is very rare. In animals with congenital lymphangiectasia or in those with disruption of the thoracic duct, chylothorax has also been described.
- Vomiting, lethargy and anorexia are uncommon clinical signs.
Laboratory Tests
Several parameters may be altered on haematological or biochemical analysis of blood samples.
Haematology
Lymphopaenia occurs as lymphocytes are the major type of cell present in lymph and they are therefore lost into the intestinal lumen in large numbers. If an inflammatory process (such as lipogranulomatous lymphangitis) has developed, there may be a monocytosis or neutrophilia.
Biochemistry
Changes on biochemistry mainly reflect the loss of lymph into the intestine:
- Panhypoproteinaemia occurs in most forms of protein-losing enteropathy and suggests that both plasma albumin and globulin are being lost.
- Hypocholesterolaemia and a reduction in the circulating concentration of triglycerides occur as these nutrients are lost into the intestinal lumen.
- Hypocalcaemia occurs due to hypoproteinaemia (reducing the total but not ionised calcium concentration) and due to vitamin D and calcium malabsorption. Hypocalcaemic tetany may be observed in animals which are severely hypocalcaemic and which then become stressed or excited.
- Hypomagnesaemia may also develop due to malabsorption but this is rarely recognised in clinical practice.
- Changes associated with SIBO are discussed here.
Other Tests
Further tests may be used to confirm the presence of protein-losing enteropathy, including measurement of faecal alpha-1 protease inhibitor concentration and faecal 51-Chromium albumin concentration after intra-venous injection.
Diagnostic Imaging
Ultrasonography
Ultrasound scans may reveal the presence of effusions (pleural fluid or ascites) and may be used to rule out other causes of PLE. The mucosa of affected intestinal loops may appear to be thickened and may also appear to have 'tiger stripes', although the latter finding is an unreliable indicator of lymphangiectasia.
Endoscopy
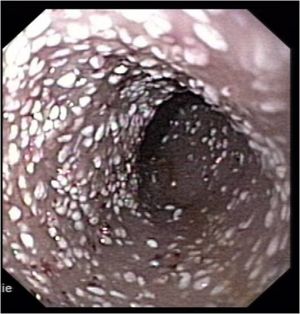
Grossly, multiple white lipid droplets can be seen to protrude from prominent mucosal blebs in the intestine (see image). The mucosa is frequently oedematous.
Histopathology
Preferably, a full thickness intestinal biopsy should be taken to achieve a definitive diagnosis. Care should be taken as hypoproteinaemic animals are at much greater risk of dehiscence at the biopsy sites, potentially leading to an acute septic peritonitis. On histological examination of the biopsy sample, accumulation of lipid-laden macrophages may be detected together with a granulomatous response around distended lymphatics.
It is essential to distinguish a true lymphangiectasia from secondary lacteal dilation that occurs with Inflammatory Bowel Disease (IBD). In the case of IBD, an inflammatory infiltrate will be seen in the lamina propria but the degree of infiltration may be underestimated if oedema is present.
Treatment
If the lymphangiectasia is secondary to another disease, the underlying cause should be treated. Otherwise, the following elements should be considered in designing a treatment plan.
Dietary modification
The diet should have a low fat content to reduce the production of lymph but should have a high calorie content to allow the animal to regain weight. The fat soluble vitamins (K, E, D and A) should be supplemented and additional calcium should be added if hypocalcaemia is documented.
Immunosuppressive
Immunosuppressive agents are a key element in the treatment of lymphangiectasia. Corticosteroids such as prednisolone are used most commonly for this pupose at an immunosuppressive dose (of 1-2 mg/kg/day in dogs). These drugs are likely to be of most benefit in those animals that have evidence of inflammatory pathology, such as lipogranulomatous lymphangitis and inflammatory infiltration of the lamina propria. If further immunosuppression is considered necessary or if adverse effects occur with corticosteroid therapy, azathioprine or ciclosporin could also be used.
Antimicrobials
Metronidazole or tylosin may be used to control any secondary SIBO. Antibiotics are thought to have effects on both the intestinal immune system and the normal enteric flora.
Fluid therapy
Short term treatment with plasma or colloids can be instituted in severely hypoproteinaemic animals that have begun to develop clinical signs. Diuretics such as frusemide and spironolactone may also be used to manage effusions.
Prognosis
The long-term prognosis is guarded as, although animals may respond to medical therapy initially, they frequently relapse and develop clinical signs associated with hypoproteinaemia.
References
- Ettinger, S.J. and Feldman, E. C. (2000) Textbook of Veterinary Internal Medicine Diseases of the Dog and Cat Volume 2 (Fifth Edition) W.B. Saunders Company.
- Hall, E.J, Simpson, J.W. and Williams, D.A. (2005) BSAVA Manual of Canine and Feline Gastroenterology (2nd Edition) BSAVA
- Nelson, R.W. and Couto, C.G. (2009) Small Animal Internal Medicine (Fourth Edition) Mosby Elsevier.