Hyperadrenocorticism
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
Also known as: Cushings Disease
Introduction
Hyperadrenocorticism is a common disease of adrenal hyperfunction that is seen most commonly in the dog. There are three known causes of the adrenal hyperfunction: dysfunction of the pituitary gland, dysfunction of the adrenal glands and iatrogenic administration of corticosteroids.
Pituitary Dependant Hyperadrenocorticism
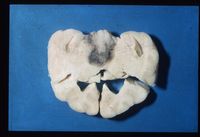
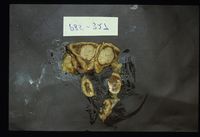
80-85% cases of Cushings disease show bilateral adrenal hyperplasia due to excess stimulation by adrenocorticotrophic hormone (ACTH). There is a failure of the negative feedback mechanism at the level of the pituitary and so ACTH is produced in an unregulated fashion. This is thought to occur due to a functional chromophobe cell (Produces ACTH and MSH) neoplasia, although visible macroadenomas are only found in 10-15% cases with this aetiology. Most cases are therefore thought to be microadenomas and may be visualised by histopathological staining of the pituitary.
Grossly the adrenals have an irregular surface with protruding nodules of cortical tissue; the hyperplased zona fasciculata cells.
Adrenal Dependant Hyperadrenocorticism
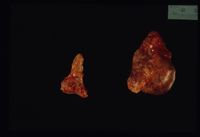
Approximately 15% all cases of Cushings disease fit into this category, which is primarily a neoplastic abnormality of the adrenal glands; approximately 50% are benign and 50% are malignant.
Iatrogenic Hyperadrenocorticism
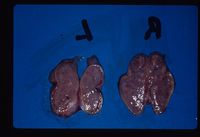
This is created by the administration of parenteral corticosteroids.In these cases adrenal atrophy is induced as the administered steroids have a negative feedback effect on the pituitary and normal ACTH release is inhibited.
Clinical Signs
Cortisol interferes with antidiuretic hormone(ADH) action on the kidney resulting in polyuria and polydipsia. This, along with panting and lethargy, are the most commonly seen clinical signs of hyperadrenocortiscm.
Liver: Cortisol induces enzymes to increase gluconeogenesis leading to hyperglycaemia in Cushings patients. The pancreas attempts to maintain normal blood glucose by producing increasing amount of insulin. Eventually the pancreas is exhausted inducing a diabetic state. Clinical signs will include hepatomegaly and abdominal enlargement, which may take the appearance of overall obesity.
Cortisol stimulates protein catabolism for gluconeogenesis so patients exhibit muscle weakness. Protein catabolism and weakness gives poor wound healing. Collagen damage due to protein catabolism allows the deposition of calcium in the skin. Calcium acts as a foreign body producing a granulomatous reaction. This is called Calcinosis Cutis.
MSH production stimulates the pigmentation of the skin with melanin. Cortisol inhibits the growth phase of the hair cycle (ANAGEN) so hair growth stops. When epilated over areas of high friction there will be bilaterally symmetric non-pruritic alopecia.
Lipolysis is stimulated by cortisol to provide precursors for gluconeogenesis. Seen clinically as a redistribution of fat to the abdomen and back of the neck; pot-bellied appearance.
Immune system: cortisol is anti-inflammatory by a number of mechanisms e.g. stabilising lysosomal membranes. The circulation will contain fewer lymphocytes and eosinophils.
Diagnosis
Clinical pathology results may lead to an indicative diagnosis. Roughly 85- 95% of cases will show lymphocytopenia and eosinopenia and marked elevation of plasma alkaline phosphatase (ALP). Hypercholoesterolaemina has also been identified in 90% of dogs with this condition. Hyperglycaemia and hypernatraemia are sometimes noted, as is an increased urinary cortisol: creatinine ratio.
Other specific diagnostic procedures include: ACTH Stimulation test: measure cortisol before and 30-60 mins after i/v Synacthen administration. A positive result is initially high cortisol followed by a markedly elevated cortisol after stimulation (>600nmol/l).
Adrenal dependant disease may be unresponsive to ACTH.
Low dose dexamethasone test: Will reliably differentiate between normal and hyperadrenocorticism dogs. Sample before and 3 and 8 hours after i/v dexamethasone at 0.01mg/kg. Normal and dogs with pituitary dependant disease show suppression of cortisol production to <50% at 3 hours whereas dogs with adrenal dependant disease have high cortisol levels which are not suppressed.
High dose dexamethasone test: Distinguishes pituitary and adrenal dependant disease once Cushings has been diagnosed. Dexamethasone dose is 0.1mg/kg. Pituitary dependant disease will show suppression of cortisol production due to negative feedback at the pituitary whereas adrenal dependant disease will not.
Ultrasound of the adrenal glands can also be performed to distinguish if one is large or if both are bilaterally enlarged. This would distinguish from PDH (bilateral) and an adrenal tumour (one enlarged).
Treatment
Mitotaneelectively destroys the zona fasciculata and zona reticularis while sparing the zona glomerulosa. Mitotane will detroy the hyperplastic adrenal cortex and the remaining tissue will then provide normal plasma cortisol concentrations. The animal will no longer be able to raise its plasma cortisol above around 20- 50 nmol/L, which will provide significant clinical improvement. You may need to use an induction dose for 5- 7 days follwed then by a maintenence dose of twice a week administration.
Animals that are treated with mitotane cannot mount a sufficient immune response in cases of stress, trauma or illness. As a consequence, from the onset of mitotane treatment, prednisolone should always be prescribed so that in these cases the owner can administer the prednisolone whilst awaiting veterinary assistance.
Trilostane is a synthetic steroid analogue. It competitively inhibits enzymes of steroid synthesis and can be used as a treatment option. Trilostanes safety has been questioned and it is therefore not readily used in dogs in the UK.
L-Deprenylis a monoamine oxidase inhibitor. Increases dopamine input to hypothalamus and pituitary and so inhibits ACTH secretion.
Pituitary tumours may be treated with radiotherapy or surgery. A bilateral adrenalectomy can be performed in some cases. Hypophysectomy has been performed in some countries with good success rates. This involves removal of the gland through the nose and must be performed in a specialist referral centre.
References
Blood, D.C. and Studdert, V. P. (1999) Saunders Comprehensive Veterinary Dictionary (2nd Edition), Elsevier Science.
Church, D (2008) Endocrine System Stugy Guide, Royal Veterinary College.
Ettinger, S.J. and Feldman, E. C. (2000) Textbook of Veterinary Internal Medicine Diseases of the Dog and Cat Volume 2 (Fifth Edition), W.B. Saunders Company.
Ettinger, S.J, Feldman, E.C. (2005) Textbook of Veterinary Internal Medicine (6th edition, volume 2), W.B. Saunders Company.
Fossum, T. W. et. al. (2007) Small Animal Surgery (Third Edition), Mosby Elsevier.
Foster, A, and Foll, C. (2003) BSAVA small animal dermatology (second edition), British Small Animal Veterinary Association.
Merck & Co (2008) The Merck Veterinary Manual (Eighth Edition), Merial.
Nelson, R.W. and Couto, C.G. (2009) Small Animal Internal Medicine (Fourth Edition), Mosby Elsevier.