Leishmania
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
|
|
Leishmania
- Leishmania spp. are intracellular parasites of macrophages
- Are closely related to Trypanosoma spp.
- Cause diseases in humans, dogs and wild animals
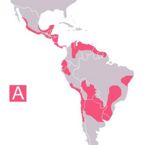
- Present in southern Europe, Africa, Asia and South America
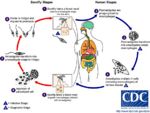
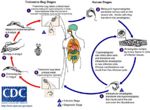
Life Cycle
- Transmitted by blood sucking sand flies
- Phlebotomus spp. in the Old World
- Lutzomyia spp. in the New World
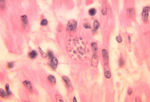
- The amastigote (morphological form) in found in vertebrate macrophages
- Multiplies and migrates to insect proboscis
- Innoculated during feeding
- Can be transmitted percutaneously if sand fly crushed on skin
- Invades macrophages and reverts to amastigote
Pathogenesis
- Infection of vertebrate host
- Produces foci of proliferating Leishmania-infected macrophages in skin (cutaneous) or internal organs (visceral)
- Very long incubation periods
- Months to years
- Many infected dogs are asymptomatic
- Cutaneous form
- Produces areas of ulceration on pinnae of ears
- Visceral form causes chronic wasting condition
- Generalised eczema
- Loss of hair around eyes producing 'spectacle' effect
- Intermittent fever
- Generalised lymphadenopathy
- Generalised eczema
- Involved in skin infections
Epidemiology
- Disease dependent on sand fly vectors
- E.g. Common in dogs around the Mediterranean coast, foci around southern Europe and around Madrid
- Reservoirs of infection
- E.g. Wild animals such as rodents and stray dogs
- Mechanisms of transmission
- Direct contact
- sand fly bite
- Leishmaniasis in British dogs
- Susceptible to infection if exposed whilst abroad in endemic areas as have no immunity
- No sand flies in Britain but dogs have become infected whilst in contact with infected imported animals
Diagnosis
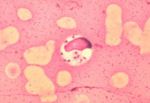
- Demonstrate Leishmania organisms
- In skin scraping or smears
- In lymph node or bone marrow biopsies
Treatment and Control
- Chemotherapy
- Prolonged treatment, expensive, suppresses infection
- Does not cure infection
- Prevent sand flies biting
- Collars, sprays containing insecticide with repellent effect
- Destruction of infected and stray dogs
- Sand flies biting infected dogs may spread the disease to to other dogs, humans and wildlife
- There is a slight possibility of transmission to humans by direct contact
Trypanosoma
- Protozoal parasites found in the blood and tissues of vertebrates
- Worldwide distribution
- Causes sleeping sickness in humans
- Particularly affect sub-Saharan Africa
- Affect cattle production
- Cause Nagana (depression)
- Divided into two groups depending on the mode of development of the insect vector
- Salivarian
- Multiply in the foregut and proboscis
- Transmitted via innoculation via feeding
- Transmitted by Tsetse flies
- Also known as anterior station development
- Stercorarian
- Multiply in the hindgut
- Infective forms migrate to the rectum
- Transmitted via contamination of wounds with insect faeces
- Also known as posterior station development
- Salivarian
- All Trypansomes except for T. equiperdum have arthropod vectors
- T. equiperdum is a venerally transmitted disease
- Non-cyclical transmission can also occur
- Mechanical transmission
- Transferred by interrupted feeding from one host to another
- Usually transmitted by biting flies, e.g. Tabanidae and Stomoxys
Recognition
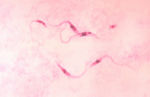
- Elongated, spindle shaped protozoa
- Between 8 and 39 μm in length
- Flagellate
- Flagellum runs the length of the body attached to the pellicle which forms an undulating membrane
- Kinetoplast present which contains the DNA of the single mitochondrion
Life Cycle
- Undergo morphological transformations in intermediate host before becoming infective for the next host
- Blood-sucking flies ingest trypanosomes whilst taking a blood meal from an infected animal
- Trypanosomes multiply first in the gut of the fly
- Salivarian trypanosomes are transmitted by Tsetse flies
- Trypanosomes pass foward to the salivary glands where they transform into the infective stage
- Innoculated with saliva when Tsetse fly next feeds on a host
- Stercorarian trypanosomes are transmitted by triatomid bugs, tabanids and keds
- Trypanosomes pass back to the rectum
- Next host is infected when skin wounds are contaminated with infected insect faeces
Pathogenesis
- Salivarian
- Causes wasting disease in cattle (nagana)
- Sleeping sickness in humans
- Stercorarian
- T. cruzi most important in veterinary medicine
- Occurs in South America
- Infects armadillos, possums and humans
- Causes Chagas Disease
- Transmitted by a triatomid (kissing) bug
- Chronic infections are often fatal causing heart failure
- Non-pathogenic species are transmitted by tabanids and keds
- T. theileria and T. melophagium
- T. cruzi most important in veterinary medicine
- Enlarged lymph nodes and spleen
- Causes lymphoid exhaustion
- Associated with plasma cell hypertrophy and hypergammaglobulinaemia
- Due to an increase in IgM
- With infections of increased duration, the lymph nodes and spleen shrink due to exhaustion of their cellular elements
- Anaemia
- Red blood cells are removed from circulation (haemolytic)
- Is a cardinal feature of the disease
- Degeneration and inflammation of multiple organs
- E.g. Skeletal muscle, myocardium and CNS
Clinical Signs
- In ruminants:
- Anaemia
- Enlargement of the lymph nodes
- Progressive loss of body condition
- Fever and appetite loss occur during parasite peaks
- Chronic disease which usually terminates in death of the animal if untreated
- Can cause abortion, infertility and decreased growth in herds
- In horses:
- Acute or chronic infections of T. brucei
- Oedema of the limbs and genitalia
- In pigs:
- T. congolense infections are mild or chronic
- T. simiae infections are hyperacute usually leading to death from pyrexia in a few days
- In dogs and cats:
- T. brucei and T. congolese
- Actute infections
- Fever, anaemia, myocarditis, corneal opacity
- Occasionally neurological signs present, such as increased aggression, ataxia and convulsions
Epidemiology
- Vector distribution
- Tsetse flies found in riverine, savannah and forest habitats
- Up to 20% flies infected
- Flies infected for life
- Parasite virulence
- Some parasitaemic animals survive for long periods of time
- E.g. T. brucei and T. congolense
- Increases the opportunity for infection of flies
- Some trypanosomes kill their host in 1-2 weeks
- E.g. T. vivax
- Decreases the chances of fly infection
- Trypanosomes avoid host immune defences by altering glycoprotein coat (surface antigen) before host antibody response
- Antigenic variation can occur many times over several months causes relapsing parasitaemia
- Some parasitaemic animals survive for long periods of time
- Host response
- Trypanotolerant wild animals remain parasitaemic for prolpnged periods without showing clincial signs of disease
- Cause lasting reservoirs of infection
- Most domestic livestock are susceptible to trypanosomosis
- Some local breeds of sheep, goats and cattle are trypanotolerant
- E.g. Bos indicus
- Trypanotolerant wild animals remain parasitaemic for prolpnged periods without showing clincial signs of disease
Diagnosis
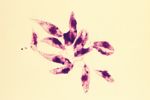
- Demonstrate trypanosomes in blood
- Giemsa stained smears
- Fresh blood films
- Motile trypanosomes
- Haematocrit tube
- Motile trypanosomes at the plasma/buffy coat interface
Control
- Tsetse fly control
- Spraying and trapping
- Prophylactic drug treatment
- Change drug group periodically to decrease the chances of resistance occuring
- May lead to protective immunity but livestock will still be susceptible to heterologous challenges
- Barrier fences and buffer zones
- Separate livestock and wild animals
- Trypanotolerant livestock
Other trypanosomes
- Mechanically transmitted by biting flies
- E.g. Surra affecting horses and camels in North Africa, Asia and South America
- T. equinum in South America
- T. evansi in Asia
- Venerally transmitted
- E.g. Dourine
- Transmitted by T. equiperdum
- Causes genital and abdominal oedema, emaciataion and CNS signs
- Affects horses and donkeys in Africa, Asia, Central and South America
- E.g. Dourine
- Non-pathogenic species occur in the UK
- In sheep caused by T. melophagium
- In cattle caused by T. theileri