Difference between revisions of "Neurons - Anatomy & Physiology"
| Line 41: | Line 41: | ||
| − | + | ||
* '''Structural''' | * '''Structural''' | ||
Revision as of 19:34, 24 August 2011
Introduction
Nerves allow electrical impulses to propagate along their elongated cell extensions and facilitate the transfer of information throughout the body. Neural tissue is found within the central nervous system (CNS) and the peripheral nervous system (PNS) and the composition and constituent parts of neurones and their surrounding cells differ.
CNS Neurons
Neural tissue found in the CNS is composed of gray and white matter. The gray matter includes nerve cell bodies and some short branches from these cell bodies. The white matter is composed of the long extensions of the nerve, the nerve fibres. Within the central nervous system there are two major cell types; neurons which are the "functional" cells of the central nervous system and glial cells which play a supporting role within the CNS. Within the CNS there are also a number of other cells that play a supportive role to the neurone and these include astrocytes, oligodendrocytes, microglial cells, ependymal cells and horoid plexus epithelial cells.
PNS Neurons
The PNS contains a series of paired nerves that extend from the spinal nerves originating from the spinal cord and cranial nerves that originate from the brain stem. Neurones found in the PNS are similar to those found in the CNS aside from a few subtle differences. Supportive cells that surround the nerves in the PNS are called Schwann cells (rather than Glial cells). The main neurotransmitter found in the PNS is acetylcholine.
Neurons
A typical neuron can be exmplified by a motor neuron in which the cell body of the nerve is located within the gray matter of the spinal cord and the nerve fibre, or axon, extends to the muscle. Nerve axons can be very long permitting electrical impulses to be sent over long distances throughout the body. The information below specifically regarding neurons is inter-changable between the CNS and PNS and therefore links have been provided where appropriate on this page to the PNS Structure page where information specific to the PNS can be found.
Basic Nerve Structure
The basic structure of a nerve is that of a cell body, or soma, which has a single long nerve fibre, or axon, attached at one end to the cell body and at the other end to another nerve cell body or to a structure requiring nerve impulses such as skeletal muscle. The interface between two nerves or a nerve and another structure is called the nerve synapse. The cell body of the nerve itself also contains numerous dendrites which increase it's surface area enabling other nerve axons to connect with the cell body. The cell body usually has connections with many other axons from other nerve cells with many synapses on the cell body. The axon of the nerve cell is usually surrounded by some form of insulating protection, or myelination. This protective layer is called the myelin sheath.
Soma
The soma, or cell body is the central part of the neuron and contains the nucleus of the cell, the rough and smooth endoplasmic reticulum, ribosomes and golgi apparatus. Similar processes that would be undertaken by any cell occur within the soma and due to the organelles it contains, the soma is where most protein synthesis occurs.
Dendrites
The neuron's dendrites are effectively branching extensions of the cell body. Collectively, these branching structures of the dendrites is known as the "dendritic tree". The dendritic tree is the site where input to the neuron occurs via synapses with axons from other nerve cells. In most cases nerve impulses travel from other nerve cells to the cell body and are then conducted along the nerve cell's own axon to other cell bodies. However, dendrites themselves are unable to propagate nerve impulses in the manner of axons as dendrites are unable to secrete neurotransmitters. Similarly axons do not possess the chemoreceptors that are found within the dendrites and are therefore unable to receive nerve impulses. Nerve impulses are therefore conducted in one direction only.
Axon (Nerve Fibre)
The axon is a very fine projection that can measure up to thousands of time the soma diameter in length. The axon carries nerve signals away from the soma. The structure and function of the axon is very similar between the CNS and the PNS.
This has been covered in detail within the PNS Structure and Anatomy page so for further information please click here.
Glial Cells
Glial cells make up approximately 50% of the volume of the nervous system and the number of glial cells out-numbers the nerve cells by a factor of ten to one. The primary function of glial cell is to provide support to the neuronal cells. There are several different types of glial-style cell; in the PNS these are referred to as Schwann cell whilst in the CNS they are referred to as Oligodendrocytes.
Glial cells form a protective networked layer around the neuron within the CNS and also help to maintain the fluid content of the tissue surrounding the nerve. This protective layer is referred to as the myelin sheath and axons that are wrapped within this myelin sheath are able to conduct nerve impulses at higher speeds than those that are not wrapped. During foetal development the glial cells wrap around axons numerous times and as the glial cell matures it looses the majority of it's cytoplasm. The remaining cellular structure consists of many layers of tightly packed lipid membranes around the axon.
Periodically along the surface of the wrapped glial cell there are gaps approximately every 1-2mm. Each gap allows the environment external to the myelin sheath to be exposed to the axon for approximately 1-2υm. These gaps in the sheath are called the Nodes of Ranvier and are important in the conduction of impulses along the axon.
Schwann Cell
Details regarding the Schwann cell have been covered within the PNS Structure and Anatomy page so for further information regarding Schwann cells please click here.
Oligodendrocytes
The term "oligodendrocyte" literally means a "cell with many branches". Oligodendrocyte precursors are found throughout the CNS and these precursors are post-mitotic, meaning oligodendrocytes can be replaced. Oligodendrocytes can myelinate up to 40 axons at a time. The myelin sheath formed by oligodendrocytes has numerous functions including; decreasing any ion leakage from the axon lowering the capacitance of the cell membrane and increasing impulse speed along the axon by allowing saltatory conduction of action potentials between the nodes of Ranvier.
Astrocytes
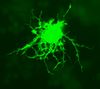
Astrocytes, or astroglia, are star-shaped glial cells within the brain which have many processes that envelope synapses made between neurons. Astrocytes have several functions including the biochemical support of the endothelial cells in forming the blood-brain barrier, provision of nutrients to nervous tissues and to form scar tissue during repair of the brain. The fluorescent image of astrocytes on the right is possible because astrocytes express glial fibrillary acidic protein (GFAP) which facilitates their identification.
- Structural
- Astrocytes are involved in the physical structuring of the brain:
- Interplay between astrocytes and mesenchymal cells is important in sculpting sulci in development.
- Astrocytes delineate the CNS by creating the CNS-PNS interface.
- The cells cover the surface of the brain.
- In this situation, the astrocytes' surface is coated by a basal lamina.
- Astrocytes take over from fibroblasts to play an important role in abscess formation, as it is difficult to induce fibrosis in the CNS.
- Astrocytes are the only differentiated CNS cell that retains the ability to divide.
- Astrocytes are involved in the physical structuring of the brain:
- Metabolic
- Astrocytes may control glucose levels and provide lactate for neurone for energy production
- This is by the lactate shuttle mechanism.
- Astrocytes have high levels of antioxidants, such as glutathione peroxidase.
- This protects neurons from damage by reactive oxygen species.
- Astrocytes may control glucose levels and provide lactate for neurone for energy production
- Blood brain barrier
- It was originally thought that the astrocyte end-feet encircling endothelial cells aided in the maintenance of the blood-brain barrier.
- The tight junctions and basal lamina of the endothelial cells are now considered to play a more substantial role in maintaining the barrier.
- It was originally thought that the astrocyte end-feet encircling endothelial cells aided in the maintenance of the blood-brain barrier.
- Transmitter reuptake
- Astrocytes:
- Isolate synapses
- Control potassium levels
- Take up transmitters
- Astrocytes express transporters for several neurotransmitters, including glutamate, ATP and GABA.
- Glutamate is particularly well taken up, and converted to glutamine using glutamine synthase.
- Astrocytes express transporters for several neurotransmitters, including glutamate, ATP and GABA.
- Astrocytes:
- Regulation of ion concentration in the extracellular space
- Astrocytes release K+ when neurons are active, increasing the local extracellular K+ concentration.
- Astrocytes have a high density of K+ channels, allowing them to rapidly clear the excess accumulation in the extracellular space.
- If this function fails extracellular K+ concentration rises. This leads neuronal depolarisation, and may be a cause of epilepsy.
- Vasomodulation
- Astrocytes may serve as intermediaries in neuronal regulation of blood flow.
- Promotion of the myelinating activity of oligodendrocytes
- Neuronal electrical activity releases ATP, which stimulates myelin formation.
- This stimulation occurs via astrocytes, which secrete LIM in response to the ATP.
- LIM is a regulatory protein that promotes the myelinating activity of oligodendrocytes.
- Control of inflammation
- Astrocytes may play a crucial rule in controlling inflammation in the CNS, by:
- Secreting chemokines
- Playing an important role in the entry of inflammatory cells to the CNS.
- When astrocytes are lost or destroyed, the neurons become very vulnerable in the face of inflammation.
- Astrocytes may play a crucial rule in controlling inflammation in the CNS, by:
Microglial Cells
Structure:
- Microglia are the CNS representatives of mononuclear phagocyte system.
- As such, they are derived from bone marrow, and are first seen in brain just before vascularistion.
- There are two populations of microglial cells:
- Extrinsic cells
- Located perivascularly and in the meninges.
- Intrinsic cells
- Located within the substance of the neural tissue.
- Extrinsic cells
Function:
- Microglia act as macrophages.
- They rapidly respond to any type of injury.
- However, microglia differ from other representatives of mononuclear phagocyte system by their low expression of MHC-2.
- MHC-2 is easily up-regulated though.
- The different types of microglia have different turnover rates.
- Perivascular and meningeal microglia turnover fairly rapidly.
- Intrinsic microglia have a very slow turnover.
Ependymal Cells
- Ependymal cells make up the is the epithelial membrane lining the ventricular system of the brain and the spinal cord.
- They are involved in the production of cerebrospinal fluid
Structure:
- Ependymal cells line the CSF-filled ventricles in the brain and the central canal of the spinal cord, and form part of the choroid plexus.
- The cells are cuboidal/columnar.
- The apical surfaces of ependymal cells are covered with:
- A layer of cilia.
- Microvilli.
Function:
- Within the ventricles, modified ependymal cells and capillaries form a system called the choroid plexus.
- The choroid plexus which produces the CSF.
- Ependymal cells beat thier cilia to circulate CSF around the central nervous system.
- The microvilli absorb CSF.
Endothelial Cells and the Blood Brain Barrier
- The walls of capillaries in the CNS are formed by endothelial cells, as for capillaries elsewhere in the body.
- However, in the CNS capillary endothelial cells are very tightly packed.
- This density of cells restricts passage of substances out of the bloodstream much more than endothelial cells in capillaries elsewhere in the body.
- This is the basis of the blood brain barrier
- This density of cells restricts passage of substances out of the bloodstream much more than endothelial cells in capillaries elsewhere in the body.
Structure and Function:
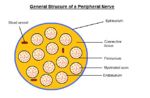
- Nerve fibres reside in a connective tissue matrix called the endoneurium and are gathered together into bundles or fascicles defined by a second connective tissue layer called the perineurium.
- Groups of fascicles are then gathered together in a third connective tissue layer called the epineurium.
- Thus, peripheral nerves have a three-tiered hierarchical arrangement of connective tissue.
- Renaut bodies are loose, cigar-shaped whorls of extracellular matrix within fascicles. They are common in horse nerves and may also occur in human and rat peripheral nerves at points of stress or compression.
Nerve Fibre
- The nerve fibre consists of the impulse-carrying axon, which is surrounded by an ensheathing cell, the Schwann cell, which in turn is surrounded by an acellular basal lamina that is continuous along the length of the nerve.
- Nerve fibres come in various discrete diameter groups, which are reflected in their conduction velocities.
- The larger the diameter the more rapid the rate of impulse conduction.
- Particular targets or receptors are associated with axons of a particular diameter, for example:
- Those connected to muscles spindles have a large diameter (20 um) and conduct at 120 m/s
- The smallest myelinated fibres are about 1um and conduct at around 6 m/s.
- The smallest fibres of all are the unmyelinated fibres (the high-threshold sensory afferents, or C-fibres, and post-ganglionic autonomies) and have a diameter of between 1 and 0.1 um. These fibres do not conduct by saltatory conduction and have very slow conduction rates of around 0.5 m/s.
The Axon
- An outer membrane called the axolemma,
- Within this there is the axoplasm which is continuous with the cytoplasm of the neuron.
- There are no ribosomes, either free or attached to endoplasmic reticulum in axons and therefore, no protein synthesis.
- Protein synthesis takes place within the cell body and some dendrites.
- All protein replacement required for the maintenance of the axon depends on proteins being imported from the cell body.
- A critical feature of the axon is its cytoskeleton, which consists of two key elements:
- The Neurofilaments
- Neurofilaments are intermediate filaments of about 10 nm diameter, and belong to the same class as other cytoskeletal proteins such as keratin, desmin, vimentin, or GFAP of astrocytes.
- Neurofilaments are formed from a triplet of polypeptide subunits of heavy (~ 200 kD), medium (~ 150 kD) and low (~ 60 kD) molecular weights.
- Typically, these subunits are heavily phosphorylated and are more numerous than microtubules, especially in large diameter axons, having a pivotal role in determining axon diameter.
- They are formed in the cell body, transported down the axon by axoplasmic transport and degraded in the terminals by Ca2+ activated proteases.
- In other words, there is a constant turnover of neurofllament within the healthy axon.
- The microtubules
- Micro tubules within axons are similar to microtubules elsewhere, consisting of polymerised dimers of alpha and beta tubulin arranged as a hollow tube of about 28 nm.
- They are relatively abundant in smaller diameter axons, and are also synthesised in the cell body.
- An important component of the cytoskeleton are the microtubule associated proteins or MAP's and the tau protein.
- These proteins are important in microtubule assembly and stability.
- Different classes of MAP's occur in the dendrites and the axons, and to some extent account for the different ultrastructural features that distinguish these two types of neuronal process.
- They form cross links between adjacent microtubules but also connect to neurofilaments and actin microfilaments, implying complex interactions between the various components of the axon skeleton.
The Schwann Cell
- Myelination in the PNS is achieved by the Schwann cell, a derivative of neural crest cells, which bud off from the neuroepithelium at a very early stage of neurogenesis.
- During development, Schwann cells engage many small axons.
- As axonal diameter increases, Schwann cells eventually relate with only a single axon c.f oligodendrocytes.
- This single axon is enveloped in a trough by the Schwann cell processes that engulf it.
- As the processes come together, an inner mesaxon is formed.
- The leading-edge process continues to move over the axon forming a spiral.
- Myelination, an extremely complex molecular process, occurs when the cytoplasm within the process is extruded allowing the internal surfaces of the membrane to come together as the major dense line, the outer membrane apposition constituting the intraperiod line.
- The alternating pattern of these two form the lamellae of compacted myelin.
- The myelin sheath is attached to, and is an integral part of, the Schwann cell on which it is dependent for its maintenance.
- A single Schwann cell forms a single myelin sheath or internode.
- There is a reasonably constant relationship between the myelin thickness and the internodal length, which in turn is associated with axon calibre.
- Large axons have long, thick myelin sheaths, and therefore also conduct more rapidly.
- The internodes do not abut one another but are separated by an exposed area of axon called the node of Ranvier.
- If the axons remain of small diameter, then a Schwann cell will continue to associate with many axons, although none of them are myelinated.
- Thus, even unmyelinated axons retain a Schwann cell ensheathment.
- These non-myelinating Schwann cells are sometimes referred to as Remak cells.
Axoplasmic Transport
- Neurons are very large cells and most of a neurons cytoplasm is present in its processes while most of the cells RNA is located in cell body (Nissl substance).
- These cells have therefore evolved mechanisms to transport large macromolecules and organelles up and down processes.
Anterograde Transport
- Two basic forms of anterograde transport can be recognised:
Fast anterograde transport
- Transports all membranous organelles such as synaptic vesicles
- Occurs at a rate of around 400mm/day (recent evidence suggests that there are many form of fast anterograde transport, mediated by different kinesins).
- Fast anterograde transport depends critically on oxidative metabolism, and is, in fact, independent of the cell body.
- The "motor" molecule is an ATPase called kinesin
Slow anterograde transport
- Deals with cytoskeletal elements and large soluble proteins.
- Slow anterograde transport can be further sub-divided into:
- A slow component, which occurs at about 2mm/day (neurofilament, rubulin, actin)
- A fast component, which occurs at around 4 mm/day, transporting all other proteins (eg myosin, clathrin).
Retrograde Transport
- Retrograde transport returns materials from the axon terminal to the cell body, either for degradation or restoration and reuse.
- As with fast anterograde transport, particles move along microtubules.
- The motor molecule for retrograde transport is dynein which is a microtubule-associated ATPase.
- The retrograde transport system is important not only for returning material to the cell body, but also provides the means whereby target-derived trophic factors, such as nerve growth factor (NGF) for dorsal root ganglion neurons, are conveyed to the cell body where they promote cell survival.