Difference between revisions of "Veterinary Education Online"
| Line 119: | Line 119: | ||
! | ! | ||
| − | <h2 style="margin:0; background:#cedff2; font-size:120%; font-weight:bold; border:1px solid #a3b0bf; text-align:left; color:#000; padding:0.2em 0.4em;">Article of the Week - [[ | + | <h2 style="margin:0; background:#cedff2; font-size:120%; font-weight:bold; border:1px solid #a3b0bf; text-align:left; color:#000; padding:0.2em 0.4em;">Article of the Week - [[Diffusion - Physiology]]</h2> |
|- | |- | ||
|style="color:#000;"| | |style="color:#000;"| | ||
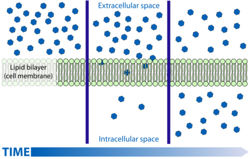
| − | [[Image: | + | [[Image:simpdifdiag.jpg|right|thumb|250px|<small><center>A Simple Schematic Diagram of Diffusion Across a Cell Membrane(Courtesy of Mariana Ruiz Villarreal)</center></small>]] |
Gases or liquids can be unevenly distributed between two areas. If one area has a higher concentration than the other then the differance between these two areas is termed the concentration gradient. The equality is then corrected by the movement of the molecules down this so called gradient from the region of high concentration to that of low. This process is passive as the molecules do not have to be forced to do this and it is reffered to as diffusion. | Gases or liquids can be unevenly distributed between two areas. If one area has a higher concentration than the other then the differance between these two areas is termed the concentration gradient. The equality is then corrected by the movement of the molecules down this so called gradient from the region of high concentration to that of low. This process is passive as the molecules do not have to be forced to do this and it is reffered to as diffusion. | ||
| Line 130: | Line 130: | ||
This works by the random thermal movement of molecules. If there is a gas present in an air tight room and then a door is opened into the next room where a lower concentration of the same gas is present the laws of probability state that more of the randomly moving molecules will escape through the door from the area of high concentration than will escape back through the door from the area of low and that eventually the concentrations in both rooms with be approximately the same. The net movement therefore will be from the room with a high concentration to that of a low concentration. | This works by the random thermal movement of molecules. If there is a gas present in an air tight room and then a door is opened into the next room where a lower concentration of the same gas is present the laws of probability state that more of the randomly moving molecules will escape through the door from the area of high concentration than will escape back through the door from the area of low and that eventually the concentrations in both rooms with be approximately the same. The net movement therefore will be from the room with a high concentration to that of a low concentration. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | [[Diffusion - Physiology|Click here to read more]] | |
| − | [[ | ||
|} | |} | ||
|- | |- | ||
Revision as of 08:39, 21 October 2008
|
Welcome to WikiVet,
A collaborative initiative between the UK Vetschools to develop a comprehensive on-line veterinary knowledge base.
5,936 articles.
| ||||||||||||||