Difference between revisions of "Phagocytosis"
Rjfrancisrvc (talk | contribs) |
Rjfrancisrvc (talk | contribs) |
||
| Line 1: | Line 1: | ||
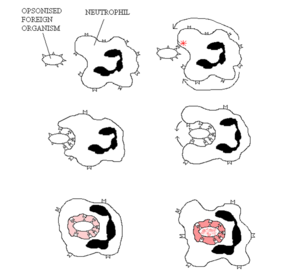
[[File:Neutrophil Phagocytosis.png|thumb|right|300px|Phagocytosis by a neutrophil]] | [[File:Neutrophil Phagocytosis.png|thumb|right|300px|Phagocytosis by a neutrophil]] | ||
| − | Phagocytosis is a very primitive system of defence against infection, having even been shown to exist in invertebrates. The process of phagocytosis itself is a form of endocytosis (cell eating), with vesicular internalisation being the method of removal of pathogens and dead cells (those that have undergone '''apoptosis''', or '''Programmed Cell Death'''). This internalised vesicle is referred to as the "'''phagosome'''". | + | Phagocytosis is a very primitive system of defence against infection, having even been shown to exist in invertebrates and single cell organisms. The discovery was made in starfish larvae by Elle Metchnikoff who subsequently won the Nobel Prize for Medicine or Physiology in 1908. The process of phagocytosis itself is a form of endocytosis (cell eating), with vesicular internalisation being the method of removal of pathogens and dead cells (those that have undergone '''apoptosis''', or '''Programmed Cell Death'''). This internalised vesicle is referred to as the "'''phagosome'''". |
Revision as of 10:37, 27 April 2012
Phagocytosis is a very primitive system of defence against infection, having even been shown to exist in invertebrates and single cell organisms. The discovery was made in starfish larvae by Elle Metchnikoff who subsequently won the Nobel Prize for Medicine or Physiology in 1908. The process of phagocytosis itself is a form of endocytosis (cell eating), with vesicular internalisation being the method of removal of pathogens and dead cells (those that have undergone apoptosis, or Programmed Cell Death). This internalised vesicle is referred to as the "phagosome".
To then either kill the pathogens or digest the dead cells, lysosomes, which contain a large range of enzymes, fuse with the phagosome to form the lysophagosome. One example of a process that occurs in these vesicles is oxygen-dependent degradation which utilizes oxygen (O2-) and chlorine (Cl*) free-radicals, hydrogen peroxide (OH), and nitric oxide (NO) to degrade the contents within the lysophagosome. The essential enzyme within this process is NADPH oxidase. In humans, the lethal genetic disease chronic granulomatous disease (CGD) is caused by the lack of NADPH oxidase in the phagocytes, with sufferers rarely living past their mid-twenties (with the help of antibiotics) usually succumbing to lung infection.
Along with the direct degradation of some elements within the lysophagosome, the process also causes acidification of the lysophagosome vesicles. The lowering of the pH then activates other enzymes within the phagosome. These include:
- Oxygen-independent proteolytic enzymes such as Defensins, Lysozyme, and cationic proteins
- Antimicrobial elements such as lactoferrin
To then complete the phagocytic process, microbes are then digested by a number of different catabolic enzymes:
- Glycosidases: Digest carbohydrates
- Lipases: Digest lipids
- Proteases: Digest protein
The waste products of phagocytosis are then either exocytosed or further degraded by the phagocyte.
The main effector cells of this process are the neutrophils and macrophages which recognise the pathogens, or with the case of macrophages, cells that have undergone apoptosis too. Phagocytic cells target pathogens by using cell membrane receptors ("Pathogen Recognition Receptors") that recognise intrinsically foreign components of microorganisms (pathogen-associated molecular patterns; PAMPs); or those that have been opsonised with either complement component C3bi or antibodies; or with the case of cells that have undergone apoptosis, externalised phosphatidylserine (a phospholipid in the cell membrane).
Links
| Originally funded by the RVC Jim Bee Award 2007 |