Difference between revisions of "Diabetes Mellitus"
| (20 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{OpenPagesTop}} |
| − | + | ==Introduction== | |
| − | == | ||
The clinical syndrome described by the term '''diabetes mellitus''' results from intolerance to glucose. It is a chronic disease caused by an absolute or relative deficiency of insulin and, although all body systems are ultimately affected, it is primarily a disorder of carbohydrate metabolism. The approximate incidence of the disease is 13 cases/10,000 dogs years at risk<ref name="one">Fall T, Hamlin HH, Hedhammar A, Kämpe O, Egenvall A. '''Diabetes mellitus in a population of 180,000 insured dogs: incidence, survival, and breed distribution.''' ''J Vet Intern Med. 2007 Nov-Dec;21(6):1209-16.''</ref>. | The clinical syndrome described by the term '''diabetes mellitus''' results from intolerance to glucose. It is a chronic disease caused by an absolute or relative deficiency of insulin and, although all body systems are ultimately affected, it is primarily a disorder of carbohydrate metabolism. The approximate incidence of the disease is 13 cases/10,000 dogs years at risk<ref name="one">Fall T, Hamlin HH, Hedhammar A, Kämpe O, Egenvall A. '''Diabetes mellitus in a population of 180,000 insured dogs: incidence, survival, and breed distribution.''' ''J Vet Intern Med. 2007 Nov-Dec;21(6):1209-16.''</ref>. | ||
===Aetiology=== | ===Aetiology=== | ||
[[Image:Diabetes Mellitus Type IV hypersensitivity.jpg|right|thumb|150px|Diabetes Mellitus-Brian Catchpole RVC 2008]] | [[Image:Diabetes Mellitus Type IV hypersensitivity.jpg|right|thumb|150px|Diabetes Mellitus-Brian Catchpole RVC 2008]] | ||
| − | Insulin is produced in the beta cells of the pancreatic islets of Langerhans and is released into the circulation to act on specific cell-surface receptors. Its release is stimulated by rising blood glucose concentration and it is principally insulin which is responsible for the post-prandial | + | [[Insulin]] is produced in the beta cells of the pancreatic islets of Langerhans and is released into the circulation to act on specific cell-surface receptors. Its release is stimulated by rising blood glucose concentration and it is principally insulin which is responsible for the post-prandial cellular glucose uptake and storage observed in humans and dogs. Several hormones (including corticosteroids, progesterone, oestrogen, growth hormone, glucagon and catecholamines) have an antagonistic effect to insulin and cause the blood glucose concentration to increase. Interruptions at any stage in this pathway may produce the clinical syndrome of diabetes mellitus, including: |
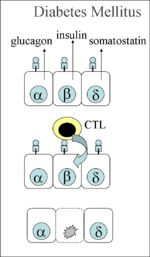
| − | *Failure to produce insulin resulting in an '''absolute deficiency''' - This may be due to [[Pancreas, Endocrine - Degenerative Pathology|degenerative changes in the beta cells]] or it may occur with severe exocrine pancreatic disease that disrupts the islets of Langerhans. The major example of the latter disease process is [[Pancreatitis | + | *Failure to produce insulin resulting in an '''absolute deficiency''' - This may be due to [[Pancreas, Endocrine - Degenerative Pathology|degenerative changes in the beta cells]] or it may occur with severe exocrine pancreatic disease that disrupts the islets of Langerhans. The major example of the latter disease process is [[Pancreatitis|pancreatitis]] and, in cases of this diesase, diabetes mellitus is often found concurrently with [[Exocrine Pancreatic Insufficiency|exocrine pancreatic insufficiency]]. Degeneration of the beta cells, whether it involves the immune system or not, results in '''type 1''' diabetes mellitus and miniature Poodles, Dachshunds and terriers appear to be predisposed to this condition. In humans, it is speculated that immune responses directed at certain pathogens (notably coxsackie virus B1) may cross-react with antigens expressed on the surface of beta cells resulting in immune-mediated destruction of these cells. Whether type 1 diabetes mellitus is associated with a similar misdirected immune response is not yet clear in small animals with several studies giving conflicting results as to the presence of autoantibodies directed at the beta cells at the point at which the disease is first diagnosed. The pathogenesis would be via [[Type IV Hypersensitivity|Type IV hypersensitivity]]. The CTLs react as if all the beta cells in the pancreas are infected by a virus, as it wrongly detects a self antigen presented by the MHC class I on the surface of the cell as foreign. Autoreactive CD8+ CTLs are inadvertently activated, destroying the beta cells, thus preventing the secretion of insulin and causing diabetes type 1 (see diagram). |
Cats may suffer from '''islet amyloidosis''' in which the protein amylin is deposited in the tissue and has directly cytotoxic effects on the beta cells. Amylin is a protein which is produced normally in the beta cells at the same rate as insulin and has synergistic effects on many aspects of metabolism. In situations where the synthesis of insulin is increased due to insulin resistance (see below), amylin is also produced in excess and it then forms aggregates that are deposited in the pancreatic tissue. | Cats may suffer from '''islet amyloidosis''' in which the protein amylin is deposited in the tissue and has directly cytotoxic effects on the beta cells. Amylin is a protein which is produced normally in the beta cells at the same rate as insulin and has synergistic effects on many aspects of metabolism. In situations where the synthesis of insulin is increased due to insulin resistance (see below), amylin is also produced in excess and it then forms aggregates that are deposited in the pancreatic tissue. | ||
| Line 35: | Line 34: | ||
Reductions in renal output allow ketone bodies and glucose to increase to ever higher concentrations in the blood. Water moves from the intracellular space to compensate for this high plasma osmolality and the alterations in cellular hydration may result in comas or seizures. | Reductions in renal output allow ketone bodies and glucose to increase to ever higher concentrations in the blood. Water moves from the intracellular space to compensate for this high plasma osmolality and the alterations in cellular hydration may result in comas or seizures. | ||
| − | The combined effects of these metabolic derangements may be life-threatening and urgent medical intervention is required. | + | The combined effects of these metabolic derangements may be life-threatening and urgent medical intervention is required. |
| + | |||
| + | '''[[Diabetic Ketoacidosis]]''' provides a complete review of the condition. | ||
====Chronic Disease==== | ====Chronic Disease==== | ||
| Line 41: | Line 42: | ||
Diabetic animals may suffer from '''peripheral neuropathies''' and '''retinopathy''' and they will have some level of '''immunosuppression'''. Affected animals are therefore predisposed to the development of chronic skin and urinary tract infections. | Diabetic animals may suffer from '''peripheral neuropathies''' and '''retinopathy''' and they will have some level of '''immunosuppression'''. Affected animals are therefore predisposed to the development of chronic skin and urinary tract infections. | ||
| + | |||
| + | |||
==Signalment== | ==Signalment== | ||
Diabetes mellitus is most common in mature dogs and it is twice as common in females than in males. Miniature Poodles, Dachshunds and terriers may suffer from degenerative changes and type 1 disease. Non-insulin dependent diabetes mellitus is paticularly common in obese indoor cats with low physical activity <ref>Slingerland LI, Fazilova VV, Plantinga EA, Kooistra HS, Beynen AC. '''Indoor confinement and physical inactivity rather than the proportion of dry food are risk factors in the development of feline type 2 diabetes mellitus.''' ''Vet J. 2009 Feb;179(2):247-53. Epub 2007 Oct 26.''</ref>. | Diabetes mellitus is most common in mature dogs and it is twice as common in females than in males. Miniature Poodles, Dachshunds and terriers may suffer from degenerative changes and type 1 disease. Non-insulin dependent diabetes mellitus is paticularly common in obese indoor cats with low physical activity <ref>Slingerland LI, Fazilova VV, Plantinga EA, Kooistra HS, Beynen AC. '''Indoor confinement and physical inactivity rather than the proportion of dry food are risk factors in the development of feline type 2 diabetes mellitus.''' ''Vet J. 2009 Feb;179(2):247-53. Epub 2007 Oct 26.''</ref>. | ||
| + | |||
| + | |||
==Diagnosis== | ==Diagnosis== | ||
| Line 93: | Line 98: | ||
An '''electrocardiogram''' should be performed in cases of DKA to assess the degree of cardiac compromise caused by hyperkalaemia. Common findings in this condition include bradycardia, reduced R wave amplitude, reduced or absent P waves, spiked T waves, a reduced Q-T interval and an increased P-R interval. | An '''electrocardiogram''' should be performed in cases of DKA to assess the degree of cardiac compromise caused by hyperkalaemia. Common findings in this condition include bradycardia, reduced R wave amplitude, reduced or absent P waves, spiked T waves, a reduced Q-T interval and an increased P-R interval. | ||
| + | |||
| + | |||
==Treatment== | ==Treatment== | ||
| Line 100: | Line 107: | ||
Animals presenting with DKA are often collapsed, comatose and severely dehydrated. Stabilisation would involve the following aspects of care: | Animals presenting with DKA are often collapsed, comatose and severely dehydrated. Stabilisation would involve the following aspects of care: | ||
*'''Intra-venous fluid therapy''' with a suitable product. The priorities of fluid therapy are to hydrate the animal and prevent further damage due to poor tissue perfusion and to provide sodium which will have been lost with the osmotic diuresis. With the latter aim in mind, 0.9% sodium chloride solution is recommended. Other clinicians prefer to use compound sodium lactate (Hartmann's solution) as it provides some buffering capacity and, because its potassium content is much lower than that of normal plasma, it is unlikely to worsen any hyperkalaemia. Fluid deficits should be replaced over 24 hours and fluids should not be infused at rates much above twice maintenance to prevent cerebral oedema for occurring due to rapid alterations in electrolyte concentrations. It would also be advisable to measure serum electrolyte concentrations regularly to prevent this effect from occurring. | *'''Intra-venous fluid therapy''' with a suitable product. The priorities of fluid therapy are to hydrate the animal and prevent further damage due to poor tissue perfusion and to provide sodium which will have been lost with the osmotic diuresis. With the latter aim in mind, 0.9% sodium chloride solution is recommended. Other clinicians prefer to use compound sodium lactate (Hartmann's solution) as it provides some buffering capacity and, because its potassium content is much lower than that of normal plasma, it is unlikely to worsen any hyperkalaemia. Fluid deficits should be replaced over 24 hours and fluids should not be infused at rates much above twice maintenance to prevent cerebral oedema for occurring due to rapid alterations in electrolyte concentrations. It would also be advisable to measure serum electrolyte concentrations regularly to prevent this effect from occurring. | ||
| − | *'''Insulin''' should be provided to reverse the metabolic changes that have resulted in the crisis. Since the administration of insulin may also have marked consequences for electrolyte status, it is best to administer it gradually as an infusion of soluble insulin. The insulin solution (made up in 0.9% sodium chloride) should be administered through a separate fluid line to that used for conventional fluids and the solution should be run through this line to saturate the bindings sites along the plastic with insulin molecules. Blood glucose concentration should be measured | + | *'''Insulin''' should be provided to reverse the metabolic changes that have resulted in the crisis. Since the administration of insulin may also have marked consequences for electrolyte status, it is best to administer it gradually as an infusion of soluble insulin. The insulin solution (made up in 0.9% sodium chloride) should be administered through a separate fluid line to that used for conventional fluids and the solution should be run through this line to saturate the bindings sites along the plastic with insulin molecules. Blood glucose concentration should be measured regularly. Alternatively, intermittent injections of insulin may be used at hourly intervals while also measuring the blood glucose concentration. |
It is important that insulin is not administered too quickly because it causes both potassium and phosphate to move intracellularly with glucose. This can result in rebound hypoglycaemia, hypokalaemia, hypophosphataemia and hypomagnesaemia because the total body levels of these cations will probably have been reduced by the enforced osmotic diuresis). Severe hypophosphataemia may result in the development of haemolytic anaemia. Potassium may need to be supplemented from the outset and the rate at which insulin is administered should be reduced if the animal is hypokalaemic on presentation. Other electrolytes should only be supplemented after their serum levels have been measured. | It is important that insulin is not administered too quickly because it causes both potassium and phosphate to move intracellularly with glucose. This can result in rebound hypoglycaemia, hypokalaemia, hypophosphataemia and hypomagnesaemia because the total body levels of these cations will probably have been reduced by the enforced osmotic diuresis). Severe hypophosphataemia may result in the development of haemolytic anaemia. Potassium may need to be supplemented from the outset and the rate at which insulin is administered should be reduced if the animal is hypokalaemic on presentation. Other electrolytes should only be supplemented after their serum levels have been measured. | ||
*'''Infections''' occur frequently, either as a cause or effect of DKA. Broad spectrum bactericidal antibiotics are generally recommended in all cases. | *'''Infections''' occur frequently, either as a cause or effect of DKA. Broad spectrum bactericidal antibiotics are generally recommended in all cases. | ||
| Line 159: | Line 166: | ||
The overall prognosis for animals with diabetes mellitus is dependent on multiple factors but the '''median survival time from diagnosis is two years in dogs''' with a relatively higher mortality in the six months following diagnosis<ref name="one">Fall T, Hamlin HH, Hedhammar A, Kämpe O, Egenvall A. '''Diabetes mellitus in a population of 180,000 insured dogs: incidence, survival, and breed distribution.''' ''J Vet Intern Med. 2007 Nov-Dec;21(6):1209-16.''</ref>. The quality of life of animals with diabetes mellitus should be monitored by both owners and veterinary surgeons. | The overall prognosis for animals with diabetes mellitus is dependent on multiple factors but the '''median survival time from diagnosis is two years in dogs''' with a relatively higher mortality in the six months following diagnosis<ref name="one">Fall T, Hamlin HH, Hedhammar A, Kämpe O, Egenvall A. '''Diabetes mellitus in a population of 180,000 insured dogs: incidence, survival, and breed distribution.''' ''J Vet Intern Med. 2007 Nov-Dec;21(6):1209-16.''</ref>. The quality of life of animals with diabetes mellitus should be monitored by both owners and veterinary surgeons. | ||
| − | == | + | {{Learning |
| − | [[ | + | |flashcards = [[Small Animal Abdominal and Metabolic Disorders Q&A 06]]<br>[[Feline Medicine Q&A 15]] |
| − | + | |powerpoints = [[E-Lecture:Canine Diabetes|Canine Diabetes e-lecture]] | |
| − | + | |literature search = [http://www.cabdirect.org/search.html?rowId=1&options1=AND&q1=%22Diabetes+Mellitus%22&occuring1=title&rowId=2&options2=AND&q2=&occuring2=freetext&rowId=3&options3=AND&q3=&occuring3=freetext&publishedstart=2000&publishedend=yyyy&calendarInput=yyyy-mm-dd&la=any&it=any&show=all&x=54&y=14 Diabetes mellitus publications since 2000] | |
| − | |||
| − | |||
| − | [http://www.cabdirect.org/search.html?rowId=1&options1=AND&q1=%22Diabetes+Mellitus%22&occuring1=title&rowId=2&options2=AND&q2=&occuring2=freetext&rowId=3&options3=AND&q3=&occuring3=freetext&publishedstart=2000&publishedend=yyyy&calendarInput=yyyy-mm-dd&la=any&it=any&show=all&x=54&y=14 Diabetes mellitus publications since 2000] | ||
[http://www.cabdirect.org/search.html?rowId=1&options1=AND&q1=%22Diabetes+Mellitus%22&occuring1=title&rowId=2&options2=AND&q2=dogs&occuring2=od&rowId=3&options3=AND&q3=&occuring3=freetext&x=38&y=9&publishedstart=&publishedend=yyyy&calendarInput=yyyy-mm-dd&la=any&it=any&show=all Diabetes mellitus in dogs publications] | [http://www.cabdirect.org/search.html?rowId=1&options1=AND&q1=%22Diabetes+Mellitus%22&occuring1=title&rowId=2&options2=AND&q2=dogs&occuring2=od&rowId=3&options3=AND&q3=&occuring3=freetext&x=38&y=9&publishedstart=&publishedend=yyyy&calendarInput=yyyy-mm-dd&la=any&it=any&show=all Diabetes mellitus in dogs publications] | ||
[http://www.cabdirect.org/search.html?q=title%3A%28%22Diabetes+Mellitus%22%29+AND+od%3A%28cats%29 Diabetes mellitus in cats publications] | [http://www.cabdirect.org/search.html?q=title%3A%28%22Diabetes+Mellitus%22%29+AND+od%3A%28cats%29 Diabetes mellitus in cats publications] | ||
| + | |full text = [http://www.cabi.org/cabdirect/FullTextPDF/2009/20093017995.pdf ''' Diabetes in cats that require immunosuppressive therapy.''' Schermerhorn, T.; Gething, M.; Jones, B.; Australian Small Animal Veterinary Association, Bondi, Australia, 33rd World Small Animal Veterinary Association Congress, Dublin, Ireland, 20-24 August 2008, 2008, pp 457-458, 5 ref.] | ||
| − | [http://www.cabi.org/cabdirect/FullTextPDF/ | + | [http://www.cabi.org/cabdirect/FullTextPDF/2007/20073161530.pdf '''Diabetes mellitus in dogs - long term management.''' Kshama, M. A.; Deepti, B. R.; Sudha, G.; Intas Pharmaceuticals Ltd, Ahmedabad, India, Intas Polivet, 2007, 8, 1, pp 117-120, 5 ref.] |
| − | [http://www.cabi.org/cabdirect/FullTextPDF/ | + | [http://www.cabi.org/cabdirect/FullTextPDF/2006/20063240193.pdf '''Recent advances in diabetes mellitus.''' Schermerhorn, T.; The North American Veterinary Conference, Gainesville, USA, The North American Veterinary Conference 2003, Small Animal and Exotics. Orlando, Florida, USA, 18-22 January, 2003, 2003, pp 310-312] |
| − | + | [http://www.cabi.org/cabdirect/FullTextPDF/2005/20053195425.pdf ''' Management of the complicated diabetic.''' Zoran, D. L.; Eastern States Veterinary Association, Gainesville, USA, Small animal and exotics. Proceedings of the North American Veterinary Conference, Volume 19, Orlando, Florida, USA, 8-12 January, 2005, 2005, pp 329-331, 4 ref.] | |
| − | + | |Vetstream = [https://www.vetstream.com/felis/Content/Disease/dis00139.asp Diabetes mellitus] | |
| − | [http://www.cabi.org/cabdirect/FullTextPDF/2005/20053195425.pdf ''' Management of the complicated diabetic.''' Zoran, D. L.; Eastern States Veterinary Association, Gainesville, USA, Small animal and exotics. Proceedings of the North American Veterinary Conference, Volume 19, Orlando, Florida, USA, 8-12 January, 2005, 2005, pp 329-331, 4 ref. | + | }} |
==References== | ==References== | ||
| Line 185: | Line 190: | ||
| + | {{review}} | ||
| + | |||
| + | {{OpenPages}} | ||
| − | [[Category:Liver_-_Degenerative_Pathology]][[Category:Cat]][[Category:Liver | + | [[Category:Liver_-_Degenerative_Pathology]][[Category:Pancreatic Diseases - Cat]][[Category:Endocrine Diseases - Cat]][[Category:Neurological Diseases - Cat]][[Category:Immunological Diseases - Cat]][[Category:Liver Diseases - Cat]][[Category:Liver Diseases - Dog]][[Category:Pancreatic Diseases - Dog]][[Category:Endocrine Diseases - Dog]][[Category:Neurological Diseases - Dog]][[Category:Immunological Diseases - Dog]] |
| − | [[Category:Cell Mediated Autoimmune Diseases]] | + | [[Category:Cell Mediated Autoimmune Diseases]][[Category:Endocrine System - Pathology]] |
[[Category:Expert_Review]] | [[Category:Expert_Review]] | ||
Latest revision as of 10:20, 21 May 2016
Introduction
The clinical syndrome described by the term diabetes mellitus results from intolerance to glucose. It is a chronic disease caused by an absolute or relative deficiency of insulin and, although all body systems are ultimately affected, it is primarily a disorder of carbohydrate metabolism. The approximate incidence of the disease is 13 cases/10,000 dogs years at risk[1].
Aetiology
Insulin is produced in the beta cells of the pancreatic islets of Langerhans and is released into the circulation to act on specific cell-surface receptors. Its release is stimulated by rising blood glucose concentration and it is principally insulin which is responsible for the post-prandial cellular glucose uptake and storage observed in humans and dogs. Several hormones (including corticosteroids, progesterone, oestrogen, growth hormone, glucagon and catecholamines) have an antagonistic effect to insulin and cause the blood glucose concentration to increase. Interruptions at any stage in this pathway may produce the clinical syndrome of diabetes mellitus, including:
- Failure to produce insulin resulting in an absolute deficiency - This may be due to degenerative changes in the beta cells or it may occur with severe exocrine pancreatic disease that disrupts the islets of Langerhans. The major example of the latter disease process is pancreatitis and, in cases of this diesase, diabetes mellitus is often found concurrently with exocrine pancreatic insufficiency. Degeneration of the beta cells, whether it involves the immune system or not, results in type 1 diabetes mellitus and miniature Poodles, Dachshunds and terriers appear to be predisposed to this condition. In humans, it is speculated that immune responses directed at certain pathogens (notably coxsackie virus B1) may cross-react with antigens expressed on the surface of beta cells resulting in immune-mediated destruction of these cells. Whether type 1 diabetes mellitus is associated with a similar misdirected immune response is not yet clear in small animals with several studies giving conflicting results as to the presence of autoantibodies directed at the beta cells at the point at which the disease is first diagnosed. The pathogenesis would be via Type IV hypersensitivity. The CTLs react as if all the beta cells in the pancreas are infected by a virus, as it wrongly detects a self antigen presented by the MHC class I on the surface of the cell as foreign. Autoreactive CD8+ CTLs are inadvertently activated, destroying the beta cells, thus preventing the secretion of insulin and causing diabetes type 1 (see diagram).
Cats may suffer from islet amyloidosis in which the protein amylin is deposited in the tissue and has directly cytotoxic effects on the beta cells. Amylin is a protein which is produced normally in the beta cells at the same rate as insulin and has synergistic effects on many aspects of metabolism. In situations where the synthesis of insulin is increased due to insulin resistance (see below), amylin is also produced in excess and it then forms aggregates that are deposited in the pancreatic tissue.
- Presence of specific antibodies in the blood that reduce the effective concentration of insulin - This is a form of immune-mediated disease that has no apparent initiating factor.
- Presence of high concentrations of hormones that are antagonistic to insulin - This occurs with many endocrine diseases that result in elevated levels of particular hormones. Examples include hyperadrenocorticism (due to corticosteroids), acromegaly (due to growth hormone) and phaeochromocytoma (due to catecholamines). Pregnancy is maintained by high blood concentrations of progesterone in small animals and this may cause gestational or type 3 diabetes and a similar phenomenon may occur during dioestrus. Iatrogenic diabetes mellitus may be induced when high doses of corticosteroids or megoestrol acetate (a synthetic progestagen) are administered. Even when the antagonisitic factor is withdrawn, the signs may remain if the islets of Langerhans are in a state of islet cell exhaustion, a form of degeneration that results from chronic hyperstimulation.
- Failure of peripheral tissues to respond to insulin, resulting in resistance - This is the cause of type 2 diabetes mellitus which is described most commonly in obese cats. This form of the disease occurs due to downregulation of insulin receptors, a process which is reversible initally. As above however, chronic hyperstimulation of the beta cells may result in islet cell exhaustion and insulin insufficiency.
- Other factors are likely to be involved in the aetiopathogenesis of the disease, including stress, concurrent illness and genetic factors, including possible associations with particular dog leucocyte antigen (DLA) haplotypes.
Classification
In humans, diabetes mellitus is traditionally classified into type 1 (caused by reduced insulin production by the beta cells) and type 2 (caused by insulin resistance). It is difficult to categorise the disease in this way in animals because the exact cause of the clinical signs and the importance of any autoimmune response are unclear. For this reason, the disease is more often divided based on clinical presentation into 'insulin dependent' or 'non-insulin dependent' forms.
Insulin dependent diabetes mellitus is the most common form of the disease, accounting for almost all cases in dogs and at least half of those in cats. In cats, it is likely that there is a continuous spectrum of disease depending on the severity of disease in the islets and the degree of insulin resistance. Animals that have suffered a severe insult to their beta cell population are likely to be insulin dependent whereas, for those with early or mild disease, the form of diabetes mellitus will depend on the degree of insulin resistance due to obesity, concurrent illness, endocrine disease or exogenous pharmaceuticals. Fluctuations in the level of this insulin resistance may alter the nature of the clinical signs observed.
Pathophysiology
Acute Disease
The deficiency or insufficiency of insulin means that peripheral tissues are not able to utilise glucose as an energetic substrate. Affected animals begin to catabolise fat and protein reserves to meet their metabolic energy requirement resulting in wastage of skeletal muscle, loss of fat reserves and overall weight loss. In spite of this weight loss, animals with diabetes mellitus have a ravenous appetite and marked polyphagia. Fatty acids are released by hydrolysation of triglycerides (lipolysis) in adipose tissue and these are converted to ketone bodies (mainly beta hydroxy-butyrate and acetoacetate) by oxidation in the liver (ketogenesis). Normally, insulin would act to limit the oxidation of fatty acids and a deficiency of the hormone therefore allows ketone bodies to be produced. The ketone bodies may be used as an energy source by many tissues.
In response to hyperglycaemia, insulin normally inhibits hepatic gluconeogenesis. When insulin is deficient, this process continues and, together with dietary glucose, this results in the hyperglycaemia observed in diabetes mellitus. As the blood glucose concentration exceeds the level at which it can be reabsorbed in the proximal convoluted tubules (the renal threshold), it is lost into the urine. This creates an osmotic diuresis as water moves by osmosis into the tubular filtrate and affected animals therefore develop polyuria and compensatory polydipsia.
Diabetic Ketoacidosis
Diabetic ketoacidosis (DKA) occurs in animals with unstable diabetes mellitus. It may occur days to months after the initial clinical signs of disease are observed and, in humans, it is related to the sudden excessive production of hormones antagonistic to insulin, chiefly glucagon. DKA may therefore be caused by entry into dioestrus or stressful events. The antagonistic hormones cause further lipolysis, ketogenesis and gluconeogenesis.
As glucose and ketone bodies exceed their respective renal thresholds, the extent of the osmotic diuresis worsens causing dehydration. Electrolytes are also lost in the urine as cations (sodium, potassium and magnesium) move to balance the negative charge of the ketone bodies. Progressive dehydration leads to reduced cardiac output, tissue perfusion and renal output.
The elevated concentration of acidic ketone bodies produces a metabolic acidosis as the buffering capacity of the plasma is overwhelmed, a phenomenon which is exacerbated by the production of lactic acid as underperfused tissues switch to anaerobic glycolysis and by the losses of extracellular fluid in vomiting and diarrhoea.
Reductions in renal output allow ketone bodies and glucose to increase to ever higher concentrations in the blood. Water moves from the intracellular space to compensate for this high plasma osmolality and the alterations in cellular hydration may result in comas or seizures.
The combined effects of these metabolic derangements may be life-threatening and urgent medical intervention is required.
Diabetic Ketoacidosis provides a complete review of the condition.
Chronic Disease
Prolonged exposure to high concentrations of glucose also has negative consequences for a number of other tissues. Cataracts develop due to changes in ocular glucose metabolism. Glucose is usually degraded to water and carbon dioxide via the conventional Ebden-Meyerhoff pathway but, when this pathway is saturated, it is also converted to fructose and sorbitol by the enzyme system aldose reductase. This sorbitol and fructose leave the lens slowly and their presence leads to the osmotic movement of water into the lens, producing a cataract.
Diabetic animals may suffer from peripheral neuropathies and retinopathy and they will have some level of immunosuppression. Affected animals are therefore predisposed to the development of chronic skin and urinary tract infections.
Signalment
Diabetes mellitus is most common in mature dogs and it is twice as common in females than in males. Miniature Poodles, Dachshunds and terriers may suffer from degenerative changes and type 1 disease. Non-insulin dependent diabetes mellitus is paticularly common in obese indoor cats with low physical activity [2].
Diagnosis
The diagnosis of diabetes mellitus may be challenging, especially in collapsed animals presenting with diabetic ketoacidosis.
Clinical signs
The following signs are common in unstabilised dogs and cats with type 1 diabetes mellitus:
- Polyuria and polydipsia because the blood glucose concentration exceeds the renal threshold. The owner may complain of nocturia.
- Polyphagia in the face of weight loss because peripheral tissues are not able to utilise blood glucose and body reserves of carbohydrate, fat and protein are degraded to meet the metabolic energy requirement. This pair of clinical signs are sometimes romantically described as resembling 'starvation in the face of plenty'.
- Muscle wasting occurs in advanced cases when body protein reserves are mobilised.
- Hepatomegaly results from increased storage of glucose as glycogen in hepatocytes. Individual hepatocytes shown signs of hydropic change or 'cloudy swelling' as they accrue increasing amounts of glycogen.
- Cataracts develop as the metabolism of the lens is altered to compensate for hyperglycaemia.
- Peripheral neuropathy, manifesting as plantigrade stance.
- Chronic or recurrent pyoderma, urinary tract infection or respiratory tract infection due to relative immunosuppression.
Older cats may present with type II diabetes mellitus and these animals are often obese.
Animals with unstable diabetes mellitus may progress into diabetic ketoacidosis, a state that requires emergency treatment. Such animals often show:
- Dehydration
- Depression, lethargy and coma
- Inappetance
- Slow, deep respiration to compensate for metabolic acidoses - so-called Kussmaul respiration.
- Vomiting and diarrhoea
- Ketotic breath, whose odour resembles that of pear drops
Many of these clinical signs are underlain by the reduced cardiac output that occurs with DKA due to the renal loss of water and electrolytes. Blood pressure and peripheral perfusion are therefore reduced, leading to eventual circulatory collapse, coma and death.
Diagnostic Imaging
Radiographs or ultrasonography of the bladder may reveal evidence of cystitis, such as a thickened bladder wall and presence of (struvite) cystoliths. Animals with diabetes mellitus, due to the high glucose concentration in their urine, may have bacterial fermentation within the bladder resulting in the formation of gas bubbles in a disease called emphysematous cystitis[3]. The gas can be detected by either imaging modality.
Pathology
Pancretic biopsies are not generally used to diagnose diabetes mellitus. On gross and histological examination of tissues from affected animals, the following changes may be observed:
- The pancreas may appear normal or reduced in size due to fibrosis.
- In cats, deposition of amyloid (aggregated plaques of the protein amylin) is often observed.
- Fatty change is consistently present in the liver and kidneys.
- In immune-mediated islet cell destruction, progressive lymphoplasmacytic infiltration and selective destruction of islet cells is observed.
- The islet cells and the epithelium of the small ducts may be vacuolated.
Laboratory Tests
The defining indicator of diabetes mellitus is persistent fasting hyperglycaemia with glycosuria. Single measurements demonstrating hyperglycaemia are not sufficient to make a diagnosis as transient hyperglycaemia frequently occurs after stress, eating or excitement, particularly in cats. Urine glucose is therefore measured to confirm the diagnosis but this test is also not sufficient in isolation because glycosuria may occur independently of hyperglycaemia in a number of other diseases, including Fanconi Syndrome and primary renal glycosuria. If there is any doubt as to the cause of hyperglycaemia, repeated measurements may be made to determine whether it persists over time or blood may be submitted for measurement of fructosamine levels (see below).
A biochemical profile may reveal mildly elevated ALP, ALT and AST due to widespread hepatic hydropic change and because hepatomegaly may also cause slight hepatic cholestasis. Affected animals may also have elevated serum concentrations of triglyceride and cholesterol due to increased lipolysis.
Animals with DKA are often severely hyperkalaemic and it may be possible to measure serum ketone bodies directly in some laboratories.
Other Tests
Fructosamine refers to the product of the non-enzymatic addition of glucose molecules to plasma proteins that are exposed to high blood glucose concentrations. Since these modifications occur slowly and because plasma proteins have a relatively long half life, the level of fructosamine gives an indication of the average blood glucose concentration over the previous 2-3 weeks. Animals with diabetes mellitus are expected to have a fructosamine concentration >500 umol/l (normal <400(-500) umol/l). Low fructosamine levels are found with insulinoma.
Glycosylated haemoglobin can be measured as a similar indicator to fructosamine as it is also formed by a non-enzymatic reaction between glucose and protein. Levels greater than 7% are supportive of a diagnosis of diabetes mellitus but the test is not widely available.
Urine samples are easy to obtain and may provide supportive evidence for a diagnosis of diabetes mellitus. Ketonuria may be apparent on a dipstick but, if this develops acutely, only beta hydroxy-butyrate will be present and this is not detected by standard dipsticks. The urine would therefore need to be oxidised by mixing with hydrogen peroxide to convert beta hydroxy-butyrate to acetoacetate. In cases of cystitis, proteinuria, haematuria and pyruia would be expected and bacteria may be observed on cytological examination of the sample.
An electrocardiogram should be performed in cases of DKA to assess the degree of cardiac compromise caused by hyperkalaemia. Common findings in this condition include bradycardia, reduced R wave amplitude, reduced or absent P waves, spiked T waves, a reduced Q-T interval and an increased P-R interval.
Treatment
Treatment is generally based on supplementing insulin and making alterations to the management of the animal that result in stabilisation of the disease. Animals with DKA require immediate stabilisation and intensive monitoring.
Stabilisation
Animals presenting with DKA are often collapsed, comatose and severely dehydrated. Stabilisation would involve the following aspects of care:
- Intra-venous fluid therapy with a suitable product. The priorities of fluid therapy are to hydrate the animal and prevent further damage due to poor tissue perfusion and to provide sodium which will have been lost with the osmotic diuresis. With the latter aim in mind, 0.9% sodium chloride solution is recommended. Other clinicians prefer to use compound sodium lactate (Hartmann's solution) as it provides some buffering capacity and, because its potassium content is much lower than that of normal plasma, it is unlikely to worsen any hyperkalaemia. Fluid deficits should be replaced over 24 hours and fluids should not be infused at rates much above twice maintenance to prevent cerebral oedema for occurring due to rapid alterations in electrolyte concentrations. It would also be advisable to measure serum electrolyte concentrations regularly to prevent this effect from occurring.
- Insulin should be provided to reverse the metabolic changes that have resulted in the crisis. Since the administration of insulin may also have marked consequences for electrolyte status, it is best to administer it gradually as an infusion of soluble insulin. The insulin solution (made up in 0.9% sodium chloride) should be administered through a separate fluid line to that used for conventional fluids and the solution should be run through this line to saturate the bindings sites along the plastic with insulin molecules. Blood glucose concentration should be measured regularly. Alternatively, intermittent injections of insulin may be used at hourly intervals while also measuring the blood glucose concentration.
It is important that insulin is not administered too quickly because it causes both potassium and phosphate to move intracellularly with glucose. This can result in rebound hypoglycaemia, hypokalaemia, hypophosphataemia and hypomagnesaemia because the total body levels of these cations will probably have been reduced by the enforced osmotic diuresis). Severe hypophosphataemia may result in the development of haemolytic anaemia. Potassium may need to be supplemented from the outset and the rate at which insulin is administered should be reduced if the animal is hypokalaemic on presentation. Other electrolytes should only be supplemented after their serum levels have been measured.
- Infections occur frequently, either as a cause or effect of DKA. Broad spectrum bactericidal antibiotics are generally recommended in all cases.
- Bicarbonate therapy is recommended by some clinicians to treat the metabolic acidosis encountered in DKA. Opinions differ as to the use of this drug and many claim that restoration of renal function will result in stabilisation of the acidosis. In any case, bicarbonate should be used with care as it may cause rebound metabolic alkalosis, paradoxical cerebral acidosis and tissue hypoxia due to a left shift of the haemoglobin-oxygen dissociation curve. Paradoxical cerebral acidosis occurs because only carbon dioxide (not bicarbonate) is able to cross the blood brain barrier.
If the animal had previously been receiving insulin therapy, the cause of the instability should be identified and managed.
Management
The non-ketotic diabetic animal can be managed as an outpatient and a great deal of the monitoring and treatment of the disease will then devolve upon the owner. In dogs, almost all cases of diabetes mellitus are insulin-dependent and insulin is therefore a necessary part of the management regime. Since many cats suffer from non-insulin dependent diabetes mellitus, they can often be managed with a change in diet and oral hypoglycaemic drugs.
Entire female animals should be neutered (as hormonal changes at dioestrus or during pregnancy may destabilise the animal) and a consistent exercise regime should be implemented.
Insulin
Recombinant insulin is available in a variety of different preparations. Soluble (or neutral) insulin is short-acting and administered by infusion but, when mixed with zinc and protamine, it has a much longer duration of action. Lente insulin (a zinc salt preparation) is used most commonly in dogs and it may be given once or twice per day. Protamine zinc insulin (PZI) is usually used in cats as this species seems to be able to degrade insulin more quickly and it has a longer duration of action than the Lente preparations. Bovine insulin is usually used in Lente preparations but, in dogs that are thought to have developed antibodies to this, porcine insulin may be used as it is homologous to canine. An initial insulin dose should be selected and this should not be changed suddenly for three days to allow the animal to respond fully. Insulin has more predictable pharmacokinetics if given intra-muscularly.
In cats, PZI is usually given once per day, followed by a meal after approximately 30 minutes. A second dose of insulin is not usually required and, if it is required, a zinc-insulin preparation should be used instead of PZI as the latter may have cumulative activity.
In dogs, once daily dosing is often used but twice daily dosing (with injections at 12 hour intervals) is also an acceptable protocol. Meals are offered 30 minutes after each injection.
Diet
Dogs
The diet fed to diabetic animals should be consistent and, at least initially, it should have a good caloric density to allow animals to regain weight. If the animal is obese at presentation, a program of controlled weight loss should be instituted so that insulin resistance is also minimised. The diet should be low in simple carbohydrates which may induce the secretion of glucagon and other hormones antagonistic to insulin. A constant feeding schedule should be maintained, with the animal usually being fed twice per day at the same times.
It has been shown that a higher dietary fibre content can help to achieve better stabilisation in cases of diabetes mellitus because this substrate forms a viscous gel in the intestine that slows the absorption of glucose[4]. This effect is greatest with soluble fibre but soluble/insoluble fibre mixes are often suitable.
Cats
Similarly to dogs, the diet should be consistent and not contain a large proportion of simple carbohydrates. In cats with suspected non-insulin dependent diabetes mellitus, obesity should be corrected as a priority to reduce insulin resistance. Emaciated cats should be fed a diet with high caloric density.
Recently, diets with high protein content have become popular in the management of diabetes mellitus in cats. These diets are used because their carbohydrate content can be reduced accordingly and because cats do not develop high peaks in post-prandial blood glucose concentration when they are fed on high protein diets. It is not clear whether supplementation of dietary fibre has any beneficial effect in cats[5].
Hypoglycaemic Drugs
Around twenty percent of diabetic cats can be managed with only dietary modification and hypoglycaemic drugs, reflecting the fact that many feline diabetics suffer reversible insulin resistance. Although several classes of drug have been suggested in the management of diabetes mellitus, only the sulphonylurea glipizide is widely used for the non-insulin-dependent form. Glipizide acts to increase the secretion of insulin from the pancreatic beta cells and its action is therefore dependent on the presence of functioning beta cells. Possible adverse effects include hypoglycaemia, vomiting, hepatotoxicity with resultant icterus. The response to glipizide is very variable between cats, with some responding well and returning to normal blood glucose concentrations and others showing no response. Glipizide is not a substitute for insulin and most cats will still require insulin therapy.
Monitoring
Since diabetes mellitus is a chronic disease, its course is best monitored by the owners of the affected animal with regular visits to a veterinary surgeon. The following variables can therefore be easily measured:
- Owner's subjective opinion of the general health of the animal. The owner may be encouraged to keep a diary documenting any episodes of concern.
- Weight, since unstable animals will lose weight.
- Ketones and glucose in urine, which can be monitored semi-quantitatively with dipsticks.
- Polyuria and poldipsia as water can be measured easily before it is offered.
Repeat measurements of blood glucose and/or fructosamine can be made when the animal is presented for re-checks. Ideally, the blood glucose concentration should be maintained slightly above the normal level and it is worth remembering that many animals suffer stress-induced hyperglycaemia when in an unfamiliar environment. If this is considered to be a problem in a particular animal, the fructosamine level will give an indication of the degree of control achieved over the previous 2-3 weeks. Blood glucose curves are not indicated unless the patient shows overt clinical signs of diabetes mellitus or if a change to the insulin dose is intended.
Instability
Animals that have previosly been stabilised may suffer bouts of overt diabetes mellitus and even deteriotrate into DKA.
Causes of Instability
There are many documented reasons for this instability, of which the most common are problems with:
- Storage and use of insulin - Insulin should be stored in a fridge and should only be retained as long as is stated on its 'use by' date. The insulin should not be shaken vigorously before use and it should be kept out of sunlight. If it is to be given by infusion, the giving set should be generously run through as the insulin molecules will adsorb to the plastic of the set.
- Administration of insulin - The insulin should be drawn up into a suitable insulin syringe and injected intramuscularly. Subcutaneous injection will result in variable pharmacokinetics.
- Response to insulin - Some animals will show signs of marked hypoglycaemia after administration of insulin but then begin to show signs of polyuria/polydipsia. If the dose is too great for the animal, antagonistic hormones will be released that will result in a rebound hyperglycaemia after a period of hypoglycaemia, a phenomenon called a Somogyi overswing. This is remedied by reducing the dose of insulin given to prevent the release of antagonistic hormones.
- Rapid metabolism of insulin - Some animals metabolise insulin faster than others and begin to show signs of diabetes mellitus before their next dose is due. If insulin is given in the morning, glycosuria will often be detected in a morning urine sample. This is managed by increasing the insulin dosage or, preferably, giving two doses of insulin twelve hours apart.
- Insulin resistance - Failure to respond to insulin may occur due to an immune response to the recombinant drug or because of some underlying disease that results in the production of factors antagonistic to insulin. If an immune response is suspected, porcine insulin should be used in preference to bovine as it is homologous to the canine molecule. Disease that cause insulin resistance are listed above but the most common causes of instability are infections (particularly cystitis or skin infections), dioestrus, pregnancy or stress. Infections should be treated and female animals should be neutered after they have been stabilised.
Investigation of Instability
A full history should be taken a complete clinical examination performed to try to determine whether any concurrent disease may be contributing to the clinical signs documented. Complete biochemical and haematological analysis of blood samples and examination of a urine sample may be indicated for the same reasons, especially to rule out the presence of a urinary tract infection.
If the animal is not thought to suffer from stress-induced hyperglycaemia, it should be hospitalised and blood glucose concentrations should be measured every two hours after insulin has been administered and food offered in the normal pattern for the patient. Hourly blood samples may be necessary in some cases as the nadir of a Somogyi overswing may occur rapidly. The pattern of change of blood glucose concentration over time should help to define the cause of the instability.
Prognosis
The overall prognosis for animals with diabetes mellitus is dependent on multiple factors but the median survival time from diagnosis is two years in dogs with a relatively higher mortality in the six months following diagnosis[1]. The quality of life of animals with diabetes mellitus should be monitored by both owners and veterinary surgeons.
| Diabetes Mellitus Learning Resources | |
|---|---|
To reach the Vetstream content, please select |
Canis, Felis, Lapis or Equis |
 Test your knowledge using flashcard type questions |
Small Animal Abdominal and Metabolic Disorders Q&A 06 Feline Medicine Q&A 15 |
 Selection of relevant PowerPoint tutorials |
Canine Diabetes e-lecture |
 Search for recent publications via CAB Abstract (CABI log in required) |
Diabetes mellitus publications since 2000 |
 Full text articles available from CAB Abstract (CABI log in required) |
Diabetes in cats that require immunosuppressive therapy. Schermerhorn, T.; Gething, M.; Jones, B.; Australian Small Animal Veterinary Association, Bondi, Australia, 33rd World Small Animal Veterinary Association Congress, Dublin, Ireland, 20-24 August 2008, 2008, pp 457-458, 5 ref. |
References
- ↑ 1.0 1.1 Fall T, Hamlin HH, Hedhammar A, Kämpe O, Egenvall A. Diabetes mellitus in a population of 180,000 insured dogs: incidence, survival, and breed distribution. J Vet Intern Med. 2007 Nov-Dec;21(6):1209-16.
- ↑ Slingerland LI, Fazilova VV, Plantinga EA, Kooistra HS, Beynen AC. Indoor confinement and physical inactivity rather than the proportion of dry food are risk factors in the development of feline type 2 diabetes mellitus. Vet J. 2009 Feb;179(2):247-53. Epub 2007 Oct 26.
- ↑ Peli A, Fruganti A, Bettini G, Aste G, Boari A. Emphysematous cystitis in two glycosuric dogs. Vet Res Commun. 2003 Sep;27 Suppl 1:419-23.
- ↑ Graham PA, Maskell E, Rawlings JM, Nash AS, Markwell PJ. Influence of a high fibre diet on glycaemic control and quality of life in dogs with diabetes mellitus. J Small Anim Pract. 2002 Feb;43(2):67-73.
- ↑ Mori A, Sako T, Lee P, Nishimaki Y, Fukuta H, Mizutani H, Honjo T, Arai T. Comparison of three commercially available prescription diet regimens on short-term post-prandial serum glucose and insulin concentrations in healthy cats. Vet Res Commun. 2009 Oct;33(7):669-80. Epub 2009 Mar 26.
- Ettinger, S.J, Feldman, E.C. (2005) Textbook of Veterinary Internal Medicine (6th edition, volume 2) Elsevier Saunders
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
Error in widget FBRecommend: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt699f4145ad0a13_55342568 Error in widget google+: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt699f4145b1d0a4_60840350 Error in widget TwitterTweet: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt699f4145b5ed34_07898462
|
| WikiVet® Introduction - Help WikiVet - Report a Problem |
- OpenPages
- Liver - Degenerative Pathology
- Pancreatic Diseases - Cat
- Endocrine Diseases - Cat
- Neurological Diseases - Cat
- Immunological Diseases - Cat
- Liver Diseases - Cat
- Liver Diseases - Dog
- Pancreatic Diseases - Dog
- Endocrine Diseases - Dog
- Neurological Diseases - Dog
- Immunological Diseases - Dog
- Cell Mediated Autoimmune Diseases
- Endocrine System - Pathology
- Expert Review