Difference between revisions of "Neurogenesis - Anatomy & Physiology"
m |
|||
| (32 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{OpenPagesTop}} |
| − | + | == Introduction == | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | The nervous system develops from '''ectoderm'''. Some of the ectoderm will also develop into the ''epidermis''. Signalling factors from elsewhere in the embryo determine which parts of the ectoderm become neural tissue or epidermis. In the anterior part of the embryo, the ectoderm forms the '''neural plate''', a flat layer of ectodermal cells lying directly above the node. The morphogenic changes from this neural plate to the nervous system is split into two parts; primary and secondary neuralation. | |
| − | + | == Neural Plate Induction == | |
| − | + | [[Image:WIKIVETformationofneuraltissue.jpg|thumb|right|350px|Formation of Neural Tissue - Copyright Sophie Stenner]] | |
| − | + | Cells of the ectoderm are directed to develop into either neural ectoderm or epidermis. | |
| − | + | === How is this choice made? === | |
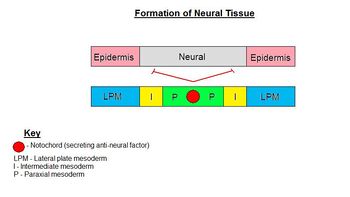
| − | + | Formation of the '''neural ectoderm''' is the default pathway. Therefore, in order to make epidermis, a signal from the environment must be diverting cells from going down this default pathway. This signal comes from the lateral plate mesoderm (LPM) which directs ectoderm to become epidermis. This signal is the '''secretory factor BMP-4'''. The notochord (lying along the midline) secretes an antagonist, Noggin, to the factor of the LPM (BMP-4), so that neural ectoderm is formed above the notochord. Therefore, in lateral regions, ectoderm becomes epidermis and along the midline ectoderm becomes neural ectoderm. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | ==Neuralation== | + | == Neuralation == |
| − | ===Primary | + | === Primary Neurulation === |
| − | + | Cells of the neural plate proliferate, causing the neural plate to thicken. Cells then converge at the midline and become wedge shaped, which drives the neural plate to become long and narrow. Cells along the midline descend ventrally and contact the notochord, forming a "hinge". This forms a depression which is called the '''neural groove'''. Either side of the neural groove, ectoderm converges towards the midline. This causes elevations either side of the neural groove, called '''neural folds'''. Hinges form at the foot of each neural fold, allowing the folds to be brought together, forming a tube. The tube sinks, as non - neural ectoderm fuses above the neural tube, "zipping" the neural tube up. The non - neural ectoderm will form epidermis. Failure of the neural tube to close causes '''spina bifida''' (spinal cord protrusion) and '''exencephaly''' (brain located out of the skull). | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | ===Secondary | + | === Secondary Neurulation === |
| − | + | Secondary neuralation occurs posteriorly, from the lumbar level onwards. The neural plate descends ventrally and medially. Cells of the neural plate condense and form a solid rod called the '''medullary cord'''. It is then made hollow by cavitations which join up to make a lumen. Tubes formed by primary and secondary neuralation fuse together to make one continuous tube called the '''neural tube'''. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | ==Neural Tube Development== | + | == Neural Tube Development == |
| − | + | [[Image:Neural tube.jpg|thumb|right|250px|Catcasillas 2010. Shh and BMP gradient in the vertebrate neural tube]] | |
| − | |||
| − | |||
| − | |||
| − | + | The neural tube is one cell thick. The lumen of the neural tube enlarges as cells at the periphery of the lumen divide under mitosis. Cell division is governed by oscillations of the nucleus. When the nucleus reaches the luminal side of cell, the cell undergoes mitosis. | |
| − | + | === Neural Tube Regionalisation === | |
| − | |||
| − | |||
| − | |||
| − | + | The neural tube undergoes regionalisation to define the anterior as the brain, and the posterior as the [[Spinal Cord - Anatomy & Physiology|spinal cord]]. In the anterior, the neural tube swells into the three ventricles of the brain: the forebrain, midbrain, and hindbrain. This occurs as fluid is pumped into the lumen of the neural tube. Pressure is regionalised to the anterior by occlusion of the neural tube at the base of the hindbrain. The occlusion reopens after enlargement of the ventricles. | |
| − | + | === Formation of Cell Layers === | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | The neural tube begins with the germinal layer which is a single cell layer thick. All cells have the capacity to undergo mitosis. Some cells then lose their ability to undergo mitosis, they detach from the germinal layer and move away from the lumen to exist behind the germinal layer. This produces new layers called the '''mantle and intermediate layer'''. The germinal layer is now known as the '''ventricular layer'''. Cells of the intermediate layer differentiate into one of two cells: | |
| − | + | #'''[[PNS Structure - Anatomy & Physiology#Nerve_Fibre|Neurones]]''' - for conductance of nerve impulses. | |
| + | #'''Glia''' - for insulation of electrical signals. | ||
| − | + | Glia form oligodendrocytes in the CNS, and [[PNS Structure - Anatomy & Physiology#Schwann Cell|schwann cells]] in the PNS. Glial cells surround the axons furthest away form the lumen of the neural tube. Because this part is myelinated, it appears ''white'' and is called the '''marginal layer'''. Axons closer to the lumen of the neural tube, which the glial cells do not surround appear ''grey''. This layer is called the '''intermediate layer'''. The ventricular layer persists, so that the spinal cord develops with these three layers. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | In the brain, further layers are added to increase complexity. This is possible because in the anterior of the embryo, neuroblasts (cells of the ventricular layer) retain their ability to undergo mitosis after leaving the ventricular layer. This difference is achieved by increased hydrostatic forces acting on the neuroblasts. | |
| − | + | == Organisation of the Nervous System == | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | + | === Somatic Nervous System === |
| − | + | Sensory neurones enter the central nervous system (CNS) through the '''dorsal root'''. Sensory neurones have their cell bodies in dorsal root ganglia (DRG). DRG are evenly spaced along the spinal cord. Response to the stimulus is mediated by a single motor neurone. Somatic motor neurones have their cell bodies in the CNS. They leave the CNS by the '''ventral root'''. Dorsal and ventral roots converge to form '''spinal nerves'''. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | + | === Autonomic Nervous System === |
| − | + | Sensory neurones also enter the CNS through the dorsal root, with their nuclei in DRG. In contrast to the somatic nervous system, the motor component is in two parts; a preganglionic and postganglionic neurone. The preganglionic neurone has its cell body in the CNS. It exits via the ventral root and synapses with the postganglionic neurone. Cell bodies of the postganglionic neurone lie outside the CNS in ganglia. Ganglia of the ''sympathetic'' nervous system are near the '''spinal cord'''. Ganglia of the ''parasympathetic'' nervous system are near the '''target organ'''. | |
| − | + | ||
| − | + | == Neural Crest == | |
| − | + | ||
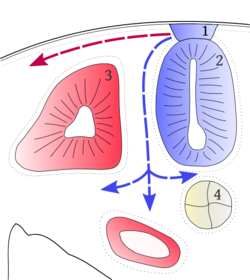
| − | + | [[Image:Neual crest.png|thumb|right|250px|Mithril 2010 Cross section scheme of vertebrate embryo. 1 – neural crest, 2 – neural tube, 3 – dorsal somite, 4 – notochord. Both paths of the neural crest cells migation marked by arrows: red one is the dorsolateral path, blue one signs the ventromedial path.]] | |
| − | + | ||
| + | The dorsal neural fold is the site where the neural ectoderm meets the epidermal ectoderm. Signaling at the border between these two tissues leads to the induction of the Neural Crest Cells, which then delaminate (epithelial to mesenchymal transition), migrate to their target sites and differentiate based on the local environmental cues (thus, their fate is plastic). The delamination and migration of neural crest cells occurs during the embryonic stage of neurulation, but is species specific and may occur while the neural tube is still open (mouse and opossum) or closed (chick). In several frog species analyzed, this migration occurs at all phases of neurulation so is not standard for any particular group of vertebrates. Migratory pathways are highly conserved across all vertebrate groups, with streams going toward the frontonasal region and branchial regions in the cranial region (with the regions lateral to the negatively numbered rhombomeres of the hindbrain free of neural crest streams). These streams are directed based on inhibitory signals in the mesenchyme (mesodermal in origin) lateral to the neural tube (rhombomere 3) or are physically blocked, such as lateral to rhombomere 5 with the otic vesicle. Apoptotic signals also play a role in the migration of these cells. The peripheral nervous system (PNS) develops from the neural crest. The cells differentiate once they reach their destination. Other non - neural tissues also develop from the neural crest. Though previously thought that only the cranial neural crest streams can form skeletal (mesodermal types) of derivatives and the trunk only neural types, recent work over the last decade has shown the extreme plasticity in cell fates of these cells along the anterior-posterior axis. Generally: | ||
| + | |||
| + | '''Anterior Neural Crest''' forms: | ||
| + | |||
| + | :sensory neurones and [[PNS Structure - Anatomy & Physiology#Schwann_Cells|schwann cells]] | ||
| + | :[[Bones - Anatomy & Physiology|bones]] of the head | ||
| + | :dermis of the face | ||
| + | :[[Cartilage - Anatomy & Physiology|cartilage]] | ||
| + | |||
| + | '''Trunk Neural Crest''' forms: | ||
| + | |||
| + | :sensory neurones of the somatic and autonomic nervous systems (ANS) | ||
| + | :sympathetic motor neurones of the ANS | ||
| + | :melanocytes | ||
| + | |||
| + | '''Posterior Neural Crest''' forms: | ||
| + | |||
| + | :parasympathetic motor neurones of the [[Alimentary System Overview - Anatomy & Physiology#Small_Intestine|gut]], which control peristalsis. | ||
| + | |||
| + | == Ectodermal Placodes == | ||
| + | |||
| + | The ectoderm thickens in places, with cells changing from cuboidal to columnar. These regions are called '''placodes'''. Placodes may be neurogenic or non - neurogenic. Neurogenic placodes are only found in the head, forming only sensory cells. Morphogenic changes produce the following derivatives: | ||
| + | |||
| + | '''Nasal placodes''' - by partial invagination. | ||
| + | |||
| + | '''Inner Ear''' - by invagination and vesicle formation. | ||
| + | |||
| + | '''Sensory Ganglion of Cranial Nerves''' - epithelial to mesenchymal transition. | ||
| + | |||
| + | {{OpenPages}} | ||
| + | [[Category:Developmental_Biology]] [[Category:Nervous_System_-_Anatomy_&_Physiology]] [[Category:A&P_Done]] [[Category:David_Hogg_reviewing]] | ||
Latest revision as of 19:04, 28 June 2012
Introduction
The nervous system develops from ectoderm. Some of the ectoderm will also develop into the epidermis. Signalling factors from elsewhere in the embryo determine which parts of the ectoderm become neural tissue or epidermis. In the anterior part of the embryo, the ectoderm forms the neural plate, a flat layer of ectodermal cells lying directly above the node. The morphogenic changes from this neural plate to the nervous system is split into two parts; primary and secondary neuralation.
Neural Plate Induction
Cells of the ectoderm are directed to develop into either neural ectoderm or epidermis.
How is this choice made?
Formation of the neural ectoderm is the default pathway. Therefore, in order to make epidermis, a signal from the environment must be diverting cells from going down this default pathway. This signal comes from the lateral plate mesoderm (LPM) which directs ectoderm to become epidermis. This signal is the secretory factor BMP-4. The notochord (lying along the midline) secretes an antagonist, Noggin, to the factor of the LPM (BMP-4), so that neural ectoderm is formed above the notochord. Therefore, in lateral regions, ectoderm becomes epidermis and along the midline ectoderm becomes neural ectoderm.
Neuralation
Primary Neurulation
Cells of the neural plate proliferate, causing the neural plate to thicken. Cells then converge at the midline and become wedge shaped, which drives the neural plate to become long and narrow. Cells along the midline descend ventrally and contact the notochord, forming a "hinge". This forms a depression which is called the neural groove. Either side of the neural groove, ectoderm converges towards the midline. This causes elevations either side of the neural groove, called neural folds. Hinges form at the foot of each neural fold, allowing the folds to be brought together, forming a tube. The tube sinks, as non - neural ectoderm fuses above the neural tube, "zipping" the neural tube up. The non - neural ectoderm will form epidermis. Failure of the neural tube to close causes spina bifida (spinal cord protrusion) and exencephaly (brain located out of the skull).
Secondary Neurulation
Secondary neuralation occurs posteriorly, from the lumbar level onwards. The neural plate descends ventrally and medially. Cells of the neural plate condense and form a solid rod called the medullary cord. It is then made hollow by cavitations which join up to make a lumen. Tubes formed by primary and secondary neuralation fuse together to make one continuous tube called the neural tube.
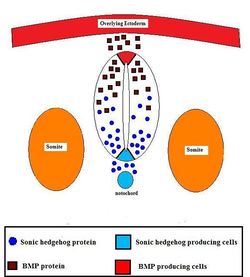
Neural Tube Development
The neural tube is one cell thick. The lumen of the neural tube enlarges as cells at the periphery of the lumen divide under mitosis. Cell division is governed by oscillations of the nucleus. When the nucleus reaches the luminal side of cell, the cell undergoes mitosis.
Neural Tube Regionalisation
The neural tube undergoes regionalisation to define the anterior as the brain, and the posterior as the spinal cord. In the anterior, the neural tube swells into the three ventricles of the brain: the forebrain, midbrain, and hindbrain. This occurs as fluid is pumped into the lumen of the neural tube. Pressure is regionalised to the anterior by occlusion of the neural tube at the base of the hindbrain. The occlusion reopens after enlargement of the ventricles.
Formation of Cell Layers
The neural tube begins with the germinal layer which is a single cell layer thick. All cells have the capacity to undergo mitosis. Some cells then lose their ability to undergo mitosis, they detach from the germinal layer and move away from the lumen to exist behind the germinal layer. This produces new layers called the mantle and intermediate layer. The germinal layer is now known as the ventricular layer. Cells of the intermediate layer differentiate into one of two cells:
- Neurones - for conductance of nerve impulses.
- Glia - for insulation of electrical signals.
Glia form oligodendrocytes in the CNS, and schwann cells in the PNS. Glial cells surround the axons furthest away form the lumen of the neural tube. Because this part is myelinated, it appears white and is called the marginal layer. Axons closer to the lumen of the neural tube, which the glial cells do not surround appear grey. This layer is called the intermediate layer. The ventricular layer persists, so that the spinal cord develops with these three layers.
In the brain, further layers are added to increase complexity. This is possible because in the anterior of the embryo, neuroblasts (cells of the ventricular layer) retain their ability to undergo mitosis after leaving the ventricular layer. This difference is achieved by increased hydrostatic forces acting on the neuroblasts.
Organisation of the Nervous System
Somatic Nervous System
Sensory neurones enter the central nervous system (CNS) through the dorsal root. Sensory neurones have their cell bodies in dorsal root ganglia (DRG). DRG are evenly spaced along the spinal cord. Response to the stimulus is mediated by a single motor neurone. Somatic motor neurones have their cell bodies in the CNS. They leave the CNS by the ventral root. Dorsal and ventral roots converge to form spinal nerves.
Autonomic Nervous System
Sensory neurones also enter the CNS through the dorsal root, with their nuclei in DRG. In contrast to the somatic nervous system, the motor component is in two parts; a preganglionic and postganglionic neurone. The preganglionic neurone has its cell body in the CNS. It exits via the ventral root and synapses with the postganglionic neurone. Cell bodies of the postganglionic neurone lie outside the CNS in ganglia. Ganglia of the sympathetic nervous system are near the spinal cord. Ganglia of the parasympathetic nervous system are near the target organ.
Neural Crest
The dorsal neural fold is the site where the neural ectoderm meets the epidermal ectoderm. Signaling at the border between these two tissues leads to the induction of the Neural Crest Cells, which then delaminate (epithelial to mesenchymal transition), migrate to their target sites and differentiate based on the local environmental cues (thus, their fate is plastic). The delamination and migration of neural crest cells occurs during the embryonic stage of neurulation, but is species specific and may occur while the neural tube is still open (mouse and opossum) or closed (chick). In several frog species analyzed, this migration occurs at all phases of neurulation so is not standard for any particular group of vertebrates. Migratory pathways are highly conserved across all vertebrate groups, with streams going toward the frontonasal region and branchial regions in the cranial region (with the regions lateral to the negatively numbered rhombomeres of the hindbrain free of neural crest streams). These streams are directed based on inhibitory signals in the mesenchyme (mesodermal in origin) lateral to the neural tube (rhombomere 3) or are physically blocked, such as lateral to rhombomere 5 with the otic vesicle. Apoptotic signals also play a role in the migration of these cells. The peripheral nervous system (PNS) develops from the neural crest. The cells differentiate once they reach their destination. Other non - neural tissues also develop from the neural crest. Though previously thought that only the cranial neural crest streams can form skeletal (mesodermal types) of derivatives and the trunk only neural types, recent work over the last decade has shown the extreme plasticity in cell fates of these cells along the anterior-posterior axis. Generally:
Anterior Neural Crest forms:
- sensory neurones and schwann cells
- bones of the head
- dermis of the face
- cartilage
Trunk Neural Crest forms:
- sensory neurones of the somatic and autonomic nervous systems (ANS)
- sympathetic motor neurones of the ANS
- melanocytes
Posterior Neural Crest forms:
- parasympathetic motor neurones of the gut, which control peristalsis.
Ectodermal Placodes
The ectoderm thickens in places, with cells changing from cuboidal to columnar. These regions are called placodes. Placodes may be neurogenic or non - neurogenic. Neurogenic placodes are only found in the head, forming only sensory cells. Morphogenic changes produce the following derivatives:
Nasal placodes - by partial invagination.
Inner Ear - by invagination and vesicle formation.
Sensory Ganglion of Cranial Nerves - epithelial to mesenchymal transition.
Error in widget FBRecommend: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt676b577f9cb634_66999707 Error in widget google+: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt676b577fa173e0_55716644 Error in widget TwitterTweet: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt676b577fa62170_87608364
|
| WikiVet® Introduction - Help WikiVet - Report a Problem |