Difference between revisions of "Toxoplasmosis - Sheep"
(Created page with '*Sheep **Mostly asymptomatic **However, if a non-immune ewe is infected during pregnancy the consequences will be serious ***Infection during the first trimester leads to resorpt…') |
|||
| (69 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {{OpenPagesTop}} | |
| − | + | ==Introduction== | |
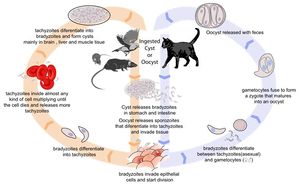
| − | + | Toxoplasmosis is the disease caused by ''[[Toxoplasma gondii]]'', an intracelluler protozoan parasite. Although the definitive host is the cat, ''T. gondii'' can infect all mammals including man and is a significant cause of abortion in sheep and goats. Toxoplasmosis does not seem to cause disease in cattle. | |
| − | + | [[Image:Toxoplasmosis Life Cycle.jpg|thumb|right|300px| Life cycle of ''Toxoplasma gondii''. Source: Wikimedia Commons; Author: LadyofHats (2010)]] | |
| − | + | ==Transmission to Sheep== | |
| − | + | ===Oocysts in the Environment=== | |
| − | + | As the definitive hosts of ''Toxoplasma gondii'', cats become infected when they hunt and eat infected wild rodents and birds. Rodents are a particularly important source of [[Toxoplasmosis - Cat and Dog|feline infection]], as they can pass ''T. gondii'' infection to their offspring without causing clinical disease. This means that a farm may develop a reservoir of ''T. gondii'' tissue cysts with the potential to cause feline infection and massive oocyst excretion when a cat is introduced to the environment. Between days 3 and 14 post-infection, cats shed over 100 million of oocysts in their faeces. Studies have shown an association between ovine toxoplasma infection, and the contamination of feed or grazing with sporulated oocysts<sup>1</sup>, highligting the importance of oocysts as a source of infection for sheep. It has also been demonstrated that the prevalence of ovine toxoplasmosis varies with the presence of cats on a farm<sup>2</sup>. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | [[Category: | + | ===Congenital Transmission=== |
| + | Apart from ingestion of oocysts in the environment, the only other method of transmission of toxoplasmosis to sheep is vertical spread from mother to foetus during pregnancy. This is because sheep are herbivorous, and do not consume animal tissues containing cysts. The outcome of transplacental infection depends on the stage of pregnancy. Infection in early gestation usually causes foetal death, as the foetal immune system is immature at this stage. In mid-gestation, infection may cause the birth of weak or stillborn lambs, sometimes accompanied by a mummified sibling. Ewes infected in the third trimester normally give birth to infected but clinically normal lambs. | ||
| + | |||
| + | ==Signalment== | ||
| + | Ovine toxoplasmosis is only clinically apparent when primary infection of a pregnant animal occurs. | ||
| + | |||
| + | ==Diagnosis== | ||
| + | A combination of clinical signs and (histo)pathology are most commonly used to make a diagnosis of ovine toxoplasmosis, but serology may be of use in some cases. | ||
| + | |||
| + | ===Clinical Signs=== | ||
| + | The signs of toxoplasmosis in sheep manifest following the exposure of a naive pregnant ewe to oocysts. The sporozoites ingested excyst in the digestive tract and penetrate the intestinal epithelium, before reaching the mesenteric lymph nodes around day 4 post-infection. Here, they cause lymphomegaly and focal necrosis before contributing to a parisitaemia from day 5. Pyrexia is associated with the development of parasitaemia. | ||
| + | |||
| + | Following dissemination of ''T. gondii'' in the blood, many tissues become infected. Parasitaemia ends when the maternal immune response becomes effective, and protozoa start to encyst as bradyzoites. In pregnant animals, the uterus is an immunoprivileged site, and the outcome of foetal infection is influenced by the stage of gestation. In early pregnancy, the foetus is unable to mount any immune response, and so cannot inhibit parasite multiplication. The foetus rapidly dies and is resorbed. In a flock, this is visible clinically as large numbers of barren ewes. In mid-gestation (70-120 days), infection can again be fatal. This causes a mummified foetus which is often twinned with a lamb that is stillborn or weak. Abortion due to infection at 70-120 days gestation tends to occur in very late pregnancy. Because the foetal immune system is well developed in late pregnancy, infection at this stage will be resisted, and the lamb will be born transiently infected but alive. | ||
| + | |||
| + | ===Laboratory Tests=== | ||
| + | Serology may be used for the laboratory diagnosis of toxoplasmosis. Ideally, the indirect fluorescent antibody test is used to detect antibody in the foetal fluids, as this is the most reliable method. If abortion products are not available, a latex agglutination test can be performed on maternal blood. Anti-''Toxoplasma gondii'' IgG antibodies can be detected in the maternal circulation from thirty days post-infection and remain increased for years afterwards. This means that for clinical diagnosis, IgG titres must be measured in paired serum samples taken 3-4 weeks apart, and must show at least a four-fold increase in titre<sup>3</sup>. In an outbreak of disease, this time scale may be too great to be useful. IgM antibodies become apparent sooner after infection and persist for a much shorter time, and so increased IgM titres are consistent with recent infection. | ||
| + | |||
| + | ===Pathology=== | ||
| + | Multiplication of ''Toxoplasma gondii'' in the placenta causes multiple foci of necrosis, which limit effective function during pregnancy. After birth, these areas of necrosis are visible as white spots on the cotyledons. The intercotyledonary areas appear normal. | ||
| + | |||
| + | Histologically, foetal tissues may display changes. In the brain, glial foci surround a necrotic centre and represent a foetal immune response to damage initiated by parasite multiplication. An associated mild lymphoid meningitis is often seen. Focal leukomalacia is also common and is thought to be due to foetal anoxia in late gestation, caused by extensive necrosis of the placentome. Focal inflammatory lesions with diffuse lymphoid infiltrates can be found in many other tissues, including the liver, lung and heart. The kidneys and skeletal muscle are less frequently affected. | ||
| + | |||
| + | ==Treatment== | ||
| + | In the event of an outbreak, little can be done to prevent further spread since transmission is via contaminated food or water rather than sheep-to-sheep contact. Since environmental contamination is related to the behaviour of cats, numbers should be limited. Keeping an older, neutered male cat may help ward of other felines: since most cats seroconvert at a young age, adults are unlikely to shed oocysts to contribute to contamination. Rodents and other vermin transmit toxoplasmosis to cats, and so populations should also be controlled. Animals should be prevented from gaining access to sheep feed or bedding. | ||
| + | |||
| + | Previously, monensin has been given in sheep feed in the lead up to lambing. Although this was shown to reduce perinatal lamb mortality related to ''Toxoplasma'' infection, monensin is no longer licensed for sheep and should not be used. | ||
| + | |||
| + | The best method of controlling ovine toxoplasmosis is therefore by vaccination. A live vaccine containing tachyzoites of the avirulent S48 strain is available. These tachyzoites do not cause pathology or form bradyzoites or tissues cysts. A single dose of vaccine is administered intramuscularly at least 3 weeks (and up to four months) prior to mating, from an age of five months. The vaccine is known to protect against toxoplasmosis for at least two lambing seasons. | ||
| + | |||
| + | ==Prognosis== | ||
| + | An outbreak of toxoplasmosis can cause significant lamb losses. However, ewes rarely show ill effects, and will not abort again in subsequent lambings. Vaccination gives excellent control of toxoplasmosis. | ||
| + | |||
| + | {{Learning | ||
| + | |literature search = [http://www.cabdirect.org/search.html?q=%28toxoplasmosis%29+AND+od%3A%28sheep%29 Toxoplasmosis in sheep publications] | ||
| + | }} | ||
| + | |||
| + | ==Links== | ||
| + | |||
| + | <big>'''[[Toxoplasmosis - Cat and Dog|Feline and Canine Toxoplasmosis]] | ||
| + | |||
| + | '''[[Toxoplasmosis - Human|Human Toxoplasmosis]]</big> | ||
| + | |||
| + | *[http://www.moredun.org.uk/feature-article.asp?ref=111 Toxoplasma infection: vaccination as a control strategy (Moredun Insititute)] | ||
| + | *[http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/52200.htm The Merck Veterinary Manual - Toxoplasmosis] | ||
| + | *[http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/110305.htm The Merck Veterinary Manual - Abortion in Sheep] | ||
| + | *[http://www.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/zoonoses/lambing.htm#toxoplasmosis Defra: Zoonoses during the lambing season] | ||
| + | |||
| + | ==References== | ||
| + | |||
| + | #Plant, J Wet al (1974) Toxoplasma infection and abortion in sheep associated with feeding of grain contaminated with cat faeces. ''Australian Veterinary Journal'', '''50''', 19–21. | ||
| + | #Skjerve, E et al (1998). Risk factors for the presence of antibodies to Toxoplasma gondii in Norwegian slaughter lambs. ''Preventative Veterinary Medicine'', '''35''', 219–227. | ||
| + | #Merck & Co (2008) '''The Merck Veterinary Manual (Eighth Edition)''' ''Merial'' | ||
| + | #Buxton, D (1990) Ovine toxoplasmosis: a review. ''Journal of the Royal Society of Medicine'', '''83''', 509-511. | ||
| + | #Innes, E A et al (2009) Ovine toxoplasmosis. ''Parastiology'', '''136''', 1887–1894. | ||
| + | #Buxton, D et all (2007) Toxoplasma gondii and ovine toxoplasmosis: New aspects of an old story. ''Veterinary Parasitology'', '''147''', 25-28. | ||
| + | #Dubey, J P (2009) Toxoplasmosis in sheep — The last 20 years. ''Veterinary Parasitology'', '''163''', 1-14. | ||
| + | |||
| + | |||
| + | {{review}} | ||
| + | |||
| + | {{OpenPages}} | ||
| + | |||
| + | [[Category:Sheep Parasites]][[Category:Reproductive Diseases - Sheep]] | ||
| + | [[Category:Brian Aldridge reviewing]] | ||
Latest revision as of 13:00, 20 July 2012
Introduction
Toxoplasmosis is the disease caused by Toxoplasma gondii, an intracelluler protozoan parasite. Although the definitive host is the cat, T. gondii can infect all mammals including man and is a significant cause of abortion in sheep and goats. Toxoplasmosis does not seem to cause disease in cattle.
Transmission to Sheep
Oocysts in the Environment
As the definitive hosts of Toxoplasma gondii, cats become infected when they hunt and eat infected wild rodents and birds. Rodents are a particularly important source of feline infection, as they can pass T. gondii infection to their offspring without causing clinical disease. This means that a farm may develop a reservoir of T. gondii tissue cysts with the potential to cause feline infection and massive oocyst excretion when a cat is introduced to the environment. Between days 3 and 14 post-infection, cats shed over 100 million of oocysts in their faeces. Studies have shown an association between ovine toxoplasma infection, and the contamination of feed or grazing with sporulated oocysts1, highligting the importance of oocysts as a source of infection for sheep. It has also been demonstrated that the prevalence of ovine toxoplasmosis varies with the presence of cats on a farm2.
Congenital Transmission
Apart from ingestion of oocysts in the environment, the only other method of transmission of toxoplasmosis to sheep is vertical spread from mother to foetus during pregnancy. This is because sheep are herbivorous, and do not consume animal tissues containing cysts. The outcome of transplacental infection depends on the stage of pregnancy. Infection in early gestation usually causes foetal death, as the foetal immune system is immature at this stage. In mid-gestation, infection may cause the birth of weak or stillborn lambs, sometimes accompanied by a mummified sibling. Ewes infected in the third trimester normally give birth to infected but clinically normal lambs.
Signalment
Ovine toxoplasmosis is only clinically apparent when primary infection of a pregnant animal occurs.
Diagnosis
A combination of clinical signs and (histo)pathology are most commonly used to make a diagnosis of ovine toxoplasmosis, but serology may be of use in some cases.
Clinical Signs
The signs of toxoplasmosis in sheep manifest following the exposure of a naive pregnant ewe to oocysts. The sporozoites ingested excyst in the digestive tract and penetrate the intestinal epithelium, before reaching the mesenteric lymph nodes around day 4 post-infection. Here, they cause lymphomegaly and focal necrosis before contributing to a parisitaemia from day 5. Pyrexia is associated with the development of parasitaemia.
Following dissemination of T. gondii in the blood, many tissues become infected. Parasitaemia ends when the maternal immune response becomes effective, and protozoa start to encyst as bradyzoites. In pregnant animals, the uterus is an immunoprivileged site, and the outcome of foetal infection is influenced by the stage of gestation. In early pregnancy, the foetus is unable to mount any immune response, and so cannot inhibit parasite multiplication. The foetus rapidly dies and is resorbed. In a flock, this is visible clinically as large numbers of barren ewes. In mid-gestation (70-120 days), infection can again be fatal. This causes a mummified foetus which is often twinned with a lamb that is stillborn or weak. Abortion due to infection at 70-120 days gestation tends to occur in very late pregnancy. Because the foetal immune system is well developed in late pregnancy, infection at this stage will be resisted, and the lamb will be born transiently infected but alive.
Laboratory Tests
Serology may be used for the laboratory diagnosis of toxoplasmosis. Ideally, the indirect fluorescent antibody test is used to detect antibody in the foetal fluids, as this is the most reliable method. If abortion products are not available, a latex agglutination test can be performed on maternal blood. Anti-Toxoplasma gondii IgG antibodies can be detected in the maternal circulation from thirty days post-infection and remain increased for years afterwards. This means that for clinical diagnosis, IgG titres must be measured in paired serum samples taken 3-4 weeks apart, and must show at least a four-fold increase in titre3. In an outbreak of disease, this time scale may be too great to be useful. IgM antibodies become apparent sooner after infection and persist for a much shorter time, and so increased IgM titres are consistent with recent infection.
Pathology
Multiplication of Toxoplasma gondii in the placenta causes multiple foci of necrosis, which limit effective function during pregnancy. After birth, these areas of necrosis are visible as white spots on the cotyledons. The intercotyledonary areas appear normal.
Histologically, foetal tissues may display changes. In the brain, glial foci surround a necrotic centre and represent a foetal immune response to damage initiated by parasite multiplication. An associated mild lymphoid meningitis is often seen. Focal leukomalacia is also common and is thought to be due to foetal anoxia in late gestation, caused by extensive necrosis of the placentome. Focal inflammatory lesions with diffuse lymphoid infiltrates can be found in many other tissues, including the liver, lung and heart. The kidneys and skeletal muscle are less frequently affected.
Treatment
In the event of an outbreak, little can be done to prevent further spread since transmission is via contaminated food or water rather than sheep-to-sheep contact. Since environmental contamination is related to the behaviour of cats, numbers should be limited. Keeping an older, neutered male cat may help ward of other felines: since most cats seroconvert at a young age, adults are unlikely to shed oocysts to contribute to contamination. Rodents and other vermin transmit toxoplasmosis to cats, and so populations should also be controlled. Animals should be prevented from gaining access to sheep feed or bedding.
Previously, monensin has been given in sheep feed in the lead up to lambing. Although this was shown to reduce perinatal lamb mortality related to Toxoplasma infection, monensin is no longer licensed for sheep and should not be used.
The best method of controlling ovine toxoplasmosis is therefore by vaccination. A live vaccine containing tachyzoites of the avirulent S48 strain is available. These tachyzoites do not cause pathology or form bradyzoites or tissues cysts. A single dose of vaccine is administered intramuscularly at least 3 weeks (and up to four months) prior to mating, from an age of five months. The vaccine is known to protect against toxoplasmosis for at least two lambing seasons.
Prognosis
An outbreak of toxoplasmosis can cause significant lamb losses. However, ewes rarely show ill effects, and will not abort again in subsequent lambings. Vaccination gives excellent control of toxoplasmosis.
| Toxoplasmosis - Sheep Learning Resources | |
|---|---|
 Search for recent publications via CAB Abstract (CABI log in required) |
Toxoplasmosis in sheep publications |
Links
Feline and Canine Toxoplasmosis
- Toxoplasma infection: vaccination as a control strategy (Moredun Insititute)
- The Merck Veterinary Manual - Toxoplasmosis
- The Merck Veterinary Manual - Abortion in Sheep
- Defra: Zoonoses during the lambing season
References
- Plant, J Wet al (1974) Toxoplasma infection and abortion in sheep associated with feeding of grain contaminated with cat faeces. Australian Veterinary Journal, 50, 19–21.
- Skjerve, E et al (1998). Risk factors for the presence of antibodies to Toxoplasma gondii in Norwegian slaughter lambs. Preventative Veterinary Medicine, 35, 219–227.
- Merck & Co (2008) The Merck Veterinary Manual (Eighth Edition) Merial
- Buxton, D (1990) Ovine toxoplasmosis: a review. Journal of the Royal Society of Medicine, 83, 509-511.
- Innes, E A et al (2009) Ovine toxoplasmosis. Parastiology, 136, 1887–1894.
- Buxton, D et all (2007) Toxoplasma gondii and ovine toxoplasmosis: New aspects of an old story. Veterinary Parasitology, 147, 25-28.
- Dubey, J P (2009) Toxoplasmosis in sheep — The last 20 years. Veterinary Parasitology, 163, 1-14.
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
Error in widget FBRecommend: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69373a70689db2_04470069 Error in widget google+: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69373a7073ed02_75931190 Error in widget TwitterTweet: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69373a707e6021_71725503
|
| WikiVet® Introduction - Help WikiVet - Report a Problem |