Digestibility of Carbohydrates
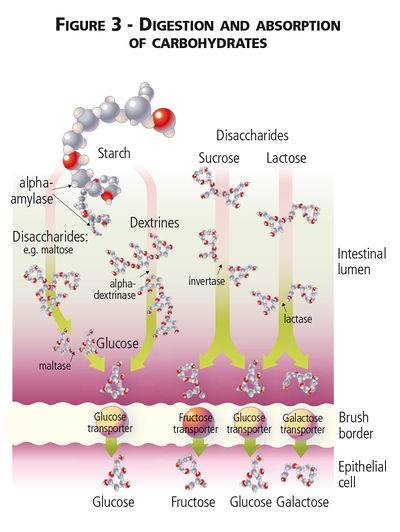
Unlike humans, dogs and cats lack salivary amylase and enzymatic digestion of carbohydrate begins in the small intestine.[1][2] The sugar alcohols mannitol, sorbitol, and xylitol are found as straight chain carbons instead of hexose (glucose and galactose) or pentose (fructose) carbon ring structures and sugar alcohols are absorbed by diffusion across the intestinal mucosa without hydrolysis.[3] Dietary monosaccharide can be absorbed directly via facilitated diffusion and Na2+-dependent glucose transporters, while disaccharide and absorbable polysaccharide carbohydrates must first be broken down by mammalian enzymes into their monosaccharide subunits.[3] Disaccharides are hydrolysed by small intestinal enzymes (maltase, sucrase and lactase) while longer chain polysaccharides (i.e. absorbable starches) must first be hydrolysed by pancreatic α-amylase. Pancreatic α-amylase breaks the α-1,4 glycosidal linkages in starch[4]. Secretion of pancreatic α-amylase, with lipase, colipase and trypsin, is under the influence of cholecystokinin (CCK), though CCK release itself is stimulated by the presence of free fatty acids and amino acids, not carbohydrates, in the duodenal lumen.[5]
Glucose, galactose and fructose, whether initially consumed as monosaccharides, disaccharides or part of a polysaccharide, are readily absorbed across the small intestinal mucosa and enter the portal circulation after meal consumption. The Na2+-dependant GLUT-1 transporter is found on small intestinal cells and facilitates transport of both glucose and galactose into the cells; fructose absorption is less well understood but is thought to involve a separate GLUT-5 transporter.[6] Absorbed glucose directly contributes to circulating blood glucose concentrations, while galactose and fructose are first metabolized by hepatic fructokinase.[7] Cats have lower concentrations of pancreatic amylase[8] as well as lower levels of hepatic glucokinase[9] relative to dogs, but are still able to digest and absorb dietary carbohydrates.[1][10] In both species, absorbed glucose can be transported directly into cells for further metabolism and oxidation to form ATP, can be used to form glycogen (the storage form of carbohydrates within animal tissues) in liver or muscle[11], or used for lipid synthesis.[12]
A number of animal factors impact carbohydrate digestion. These include age related changes in enzyme activities as well as inherent species differences in metabolic pathways. Lactase activity is highest in puppies and kittens and decreases with age[13][14]. In contrast, pancreatic amylase activity increases with age. Low fructokinse activity in cats means they can develop galactosuria or fructosuria if given these monosaccharides.[15] Overall carbohydrate digestibility decreases with age in otherwise healthy dogs and cats.[16][17]
In both dogs and cats, starch digestibility is also affected by the source and type of carbohydrate present[18] as well as the degree of processing of the carbohydrate[19][20]. The type and amount of non-absorbable carbohydrate (i.e. fibre) present in the diet will also influence the post-prandial glycaemic response in both dog and cats. The presence of highly soluble, fermentable fibre content in the diet will slow carbohydrate digestion and absorption resulting in dampened post-prandial blood glucose in both healthy[21][22] and diabetic animals.[23][24] Ground, cooked and extruded starches are almost 100% digestible in both dogs and cats[1][2][25][26], while digestibility of raw (uncooked) starches varies from 0-65% depending on the type of starch. Resistant starches are formed when solubilised dietary starch re-crystallized upon cooling forming a structure that is resistant to pancreatic amylase.[27] Undigested starches and resistant starch can then be fermented by intestinal bacteria,[28][29][30] which may contribute to clinical signs of bacterial overgrowth. Maldigestion and malabsorption of dietary starch are believed to be a feature of inflammatory bowel disease.[31]
References
- ↑ 1.0 1.1 1.2 Morris JG, et al. (1997) Carbohydrate digestion by the domestic cat (Felis catus). Br J Nutr 1997;37:365-373.
- ↑ 2.0 2.1 Hilton J. (2006) Carbohydrates in the nutrition of dog. Can Vet J 1990;46A:359-369.
- ↑ 3.0 3.1 National Research Council (NRC). (2006) Carbohydrates and Fiber. In Nutrient Requirements for Dogs and Cats. 2006 Washington, DC: National Academies Press p.51-54.
- ↑ Colonna P, et al. (1992) Limiting factors of starch hydrolysis. Eur J Clin Nutr 1992;46:S17-S32.
- ↑ Backus RC, et al. (1995) Elevation of plasma cholecystokinin (CCK) immunoreactivity by fat, protein, and amino acids in the cat, a carnivore. Regul Pept 1995;57:123-131.
- ↑ Levin RJ. (1994) Digestion and absorption of carbohydrates: From molecules and membranes to humans. Am J Clin Nutr 1994;59:690S-698S.
- ↑ Feinman RD and Fine EJ. (2013) Fructose in perspective. Nutr Metab (Lond) 2013;10:45
- ↑ McGeachin RL and Akin JR. (1979) Amylase levels in the tissues and body fluids of the domestic cat (Felis catus). Comp Biochem Physiol B 1979;63:437-439.
- ↑ Washizu T, et al. (1999) Comparison of the activities of enzymes related to glycolysis and gluconeogenesis in the liver of dogs and cats. Res Vet Sci 1999;67:205-206.
- ↑ Kienzle E. (1993) Carbohydrate metabolism in the cat. 2. Digestion of starch. JAPAN 1993;69:102-114.
- ↑ Ebiner JR, et al. (1979) Comparison of carbohydrate utilization in man using indirect calorimetry and mass spectrometry after oral load of 100 g naturally-labelled (13C) glucose. Br J Nutr 1979;41:419-429.
- ↑ Flatt JP, et al. (1985) Effects of dietary fat on postprandial substrate oxidation and on carbohydrate and fat balances. J Clin Invest 1985;76:1019-1024.
- ↑ Kienzle E. (1993) Carbohydrate metabolism in the cat. 4. Activity of maltase, isomaltase, sucrose, and lactase in the gastrointestinal tract in relation to age and diet. JAPAN 1993;70:89-96.
- ↑ Buddington RK, et al. (2003) Activities of gastric, pancreatic, and intestinal brush-border membrane enzymes during postnatal development of dogs. AJVR 2003;64:627-634.
- ↑ Kienzle E. (1994) Blood sugar levels and renal sugar excretion after the intake of high carbohydrate diets in cats. J Nut 1994;124:2563S-2567S.
- ↑ Strasser A, et al. (1993) The effect of aging on laboratory values in dogs. J Vet Med 1993;A40:720-730.
- ↑ Burkholder WJ. (1999) Age-related changes to nutritional requirements and digestive function in adult dogs and cats. Vet Med Today 1999:215:625-629.
- ↑ Bach-Knudsen KE and Hansen I. (1991) Gastrointestinal implication in pigs of wheat fractions. I. Digestibility and bulking properties of polysaccharides and other major constituents. Br J Nutr 1991;65:217-232.
- ↑ Camire ME, et al. (1990) Chemical and nutrition changes in food during extrusion. Food Sci Nutr 1990;29:35-57.
- ↑ Marsaman GJ, et al. (1997) The in vitro accessibility of untreated, toasted, and untoasted soybean meals for proteases and carbohydrases. J Ag Food Chem 1997;45:4088-4095.
- ↑ Muir HE, et al. (1996) Nutrient digestion by ileal cannulated dogs as affected by dietary fiber with various fermentation characteristics. J Anim Sci 1996;74:1641-1648.
- ↑ Nguyen P, et al. (1998) Glycemic and insulinemic response after ingestion of commercial foods in healthy dogs: Influence of food composition. J Nutr 1998;128:2654S-2658S.
- ↑ Nelson RW. (1989) The role of fiber in managing diabetes mellitus. Vet Med 1989;84:1156-1160.
- ↑ Nelson RW, et al. (2000) Effect of dietary insoluble fiber on control of glycemia in cats with naturally acquired diabetes mellitus. JAVMA 2000;216:1082-1088.
- ↑ Murray SM, et al. (1999) Evaluation of selected high-starch flours as ingredients in canine diets. J Anim Sci 1999;77:2180-2186.
- ↑ Kendall PT and Holme DW. (1982) Studies on the digestibility of soybean products, cereals, cereal and plant-based products in the diets of dogs. J Sci Food Agric 1982;33:813-822.
- ↑ Berry SC. (1986) Resistant starch: Formation and measurement of starch that survives exhaustive digestion with amylolytic enzymes during determination of dietary fiber. J Cereal Sci 1986;4:301-304.
- ↑ Rerat A, et al. (1978) Digestion and absorption of carbohydrates and nitrogenous matters in the hindgut of omnivorous nonruminant animals. J Anim Sci 1978;46:1808-1837.
- ↑ Washabau RJ, et al. (1986) Evaluation of intestinal carbohydrate malabsorption by pulmonary hydrogen gas excretion. AJVR 1986;47:1402-1406.
- ↑ Muir P, et al. (1991) Evaluation of carbohydrate malassimilation and intestinal transit time in cats by measurement of breath hydrogen excretion. AJVR 1991;52:1104-1109.
- ↑ Ugarte C, et al. (2004) Carbohydrate malabsorption is a feature of feline inflammatory bowel disease but does not increase clinical gastrointestinal signs. J Nutr 2004;134:2068S–2071S.
| This article was: Date reviewed: 18 May 2015 |
| Endorsed by WALTHAM®, a leading authority in companion animal nutrition and wellbeing for over 50 years and the science institute for Mars Petcare. |
Error in widget FBRecommend: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a2f31586a8c2_24495259 Error in widget google+: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a2f3158c2f16_93188415 Error in widget TwitterTweet: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a2f315914b72_20726879
|
| WikiVet® Introduction - Help WikiVet - Report a Problem |