Difference between revisions of "Pet Food Labels - Nutrition"
Ggaitskell (talk | contribs) |
Ggaitskell (talk | contribs) |
||
| Line 167: | Line 167: | ||
Directive 2008/38/EC lists all permitted PARNUTS (1), together with the essential characteristics (2) needed to meet the requirements for the specific PARNUT, the species (3) for which it is indicated, the additional mandatory labelling requirements (4) and other provisions where necessary (6) (Table 3). Consequently, a number of additional statements appear on the labels of dietetic pet foods. | Directive 2008/38/EC lists all permitted PARNUTS (1), together with the essential characteristics (2) needed to meet the requirements for the specific PARNUT, the species (3) for which it is indicated, the additional mandatory labelling requirements (4) and other provisions where necessary (6) (Table 3). Consequently, a number of additional statements appear on the labels of dietetic pet foods. | ||
| + | [[File:Table 3 - Pet Food Labels Nutrition Page.png|thumb|600px|left|Table 3 - Required information on labels of dietetic pet foods in Europe]] | ||
| Line 174: | Line 175: | ||
| − | |||
| − | |||
Since the legislator tends to consider most PARNUTs as being ‘temporary situations’, they make it '''mandatory to publish on the labels a defined length of time for use''' (Table 3, column 5). However, not all PARNUT indications are temporary, e.g., diabetes mellitus type 1 and chronic kidney disease are irreversible and need lifelong dietary management. This issue has been resolved by stating in column 5 ‘initially up to xx weeks or months’. In the same time this statement should stimulate the pet owner to visit the treating veterinarian for regular control check-ups. | Since the legislator tends to consider most PARNUTs as being ‘temporary situations’, they make it '''mandatory to publish on the labels a defined length of time for use''' (Table 3, column 5). However, not all PARNUT indications are temporary, e.g., diabetes mellitus type 1 and chronic kidney disease are irreversible and need lifelong dietary management. This issue has been resolved by stating in column 5 ‘initially up to xx weeks or months’. In the same time this statement should stimulate the pet owner to visit the treating veterinarian for regular control check-ups. | ||
Revision as of 08:26, 24 April 2017
Introduction
A pet food label fulfils two main roles:
- It is a document that delivers legally required information, which is important for control authorities and
- It provides information to the purchaser about the product and the producing company.
Pet food differs from human food products in that the final consumer is not the purchaser. The information on the label is provided for pet owners, caretakers or veterinarians who may choose or recommend what the cat or dog is fed. The primary purpose of a label is to provide clear, accurate and honest information on the composition, characteristics and use of the pet food product. In many countries the information included on a pet food label and the way it appears are largely defined by legislation.
The following sections describe the way pet food is labelled in the European Union and in the United States. Specific pet food labelling legislation and guidelines also exist in many other countries around the world.
Europe
In the European Union, pet food labelling is mainly governed by Regulation (EC) No 767/2009 on the Marketing and Use of Feed. This Regulation sets out the rules for feed designed for both food-producing and non-food producing animals, including requirements for labelling, packaging and claims. Regulation 767/2009 also provides a framework for the establishment and labelling of feed intended for particular nutritional purposes (dietetic feeds).
Definitions
For clarity it is important that terms used in the legislation are well defined. This is established in Article 3 of Regulation 767/2009, and some important definitions are given hereafter:
(c) Food producing animals: any animal that is fed, bred or kept for the production of food for human consumption, including animals that are not used for human consumption, but belong to a species that is normally used for human consumption in the community.
(d) Non-food producing animals: any animal that is fed, bred or kept but not used for human consumption, such as fur animals, pets and animals kept in laboratories, zoos or circuses.
(i) Complete feed means a compound feed which, by reason of its composition, is sufficient for a daily ration.
(j) Complementary feed means compound feed which has a high content of certain substances but which, by reason of its composition, is sufficient for a daily ration only if used in combination with other feeds.
(s) Labelling means the attribution of any words, particulars, trademarks, brand name, pictorial matter or symbol to a feed by placing this information on any medium like packaging, container, notice, label, document, ring, collar or the internet referring to or accompanying such feed, including for advertising purposes.
(t) Label means any tag, brand, mark, pictorial or descriptive matter, printed, stencilled, marked, embossed, impressed on, or attached to the packaging or the container, of feed.
From definitions (c) and (d) of Article 3, companion animals such as horses, pet rabbits and (racing) pigeons are considered food-producing animals by the EU Commission and the national authorities of the EU Member States.
Definition (i) does not provide any recommendation for nutrient levels that would be appropriate for a feed to be considered ‘complete’. FEDIAF’s Nutritional Guidelines list minimum recommended nutrient levels for commercial pet foods and can be used as a guidance document to determine whether or not a pet food is complete for healthy dogs and cats.
Mandatory Declarations
An example of a European label (in this case from the UK) is shown in Figure X. On the label, mandatory declarations should be easily identified and not obscured by any other information. The mandatory labelling particulars, sometimes referred to as statutory statement, must be given in their entirety set in a prominent place on the packaging or label (Regulation 767/2009 Article 14). Mandatory information must be provided in at least one of the official languages of the Member State in which it is sold.
Mandatory Labelling Requirements
Articles 15 to 20 state that the following particulars have to be declared in the statutory section of a pet food label:
Complete Feed or Complementary Feed
A pet food label must indicate whether the pet food is complete or complementary, in other words, whether the food can satisfy all nutritional demands without an additional ration (complete) or whether it must be fed with another product (complementary). The description “complete” or “complementary” must be considered in relation to the animal the food is intended for. Optionally, the life stage or purpose for the food which it is intended may be indicated (e.g., adult, growth, all life stages, activity or light). This should be clear from the label e.g., Product X is a complete pet food for adult dogs or Product Y is a complete pet food for growing and reproducing cats. Name and address of the feed business operator (company) responsible for the labelling. Approval or registration number(s), or, if not available, the address of the manufacturing plant In cases where the producer is not the person responsible for the labelling: the business name and address of the producer (e.g., private label manufacturer), or the approval number of that producer should be given.
Batch Number
This is needed for traceability. Where circumstances require it, most pet food manufacturers can trace a bag or can back to the minute it was produced.
Net Quantity
The net quantity must be expressed in units of mass (g, kg) for solid products and units of mass or volume (ml, L) for liquid products. The “e” often seen after the weight statement indicates that the product complies with all packers rules. In addition, strict rules regulate the limits of variation permitted under the declared weight to ensure that the consumer receives, on average, the amount stated on the package (Directive 2009/34).
The Minimum Storage Life
This may also be called an expiry date and requires a DD/MM/YYYY Animal species or category of animals for which the compound feed is intended E.g. adult cat, puppy etc.
Directions for Use
In practice these consist mainly of the feeding instructions, but it is not specified by law what is required as a minimum. This can also include storage instructions, especially once a pack is opened.
Composition
This is the list of feed materials (see details below).
Analytical constituents
This is the nutrient content of the feed. In Europe, the typical or proximate analysis has to be declared (see details below).
Additives (see details below)
Additional Labelling Requirement
Pet food has an additional labelling requirement in Regulation 767/2009, and that is the requirement to provide a free telephone number or other suitable means of communication to enable consumers to contact manufacturers to request additional information on the additives in the pet food and also on feed materials that are listed by category rather than single feed material.
Derogation
As a derogation, the following mandatory labelling items may be declared outside the statutory section:
- ‘best before date’,
- ‘batch number’,
- ‘net quantity’,
- ‘name and address of the feed business operator responsible for labelling’, and
- ‘approval, registration number or address of manufacturing plant’.
In such cases it shall be pointed out in the statutory section where these particulars appear.
Composition = List of Feed Materials
Feed materials (ingredients) have to be listed in descending order by weight, and the list has to be preceded by the heading: Composition. On the label of pet foods for dogs and cats, individual feed materials can be replaced by ingredient categories. These categories are designed to provide consumers with some indication of the source of raw materials used, while allowing the manufacturer some flexibility in the selection of the ingredients within a specific category (Burger ’93). The categories are defined by law in Commission Directive 82/475/EEC laying down the categories of feed materials which may be used for the purposes of labelling compound feeding stuffs for pet animals (Table 1).
Table 1: List of ingredient categories:
- Meat and animal derivatives
- Milk and milk derivatives
- Egg and egg derivatives
- Oils and fats
- Yeasts
- Fish and fish derivatives
- Cereals
- Vegetables
- Derivatives of vegetable origin
- Vegetable protein extracts
- Minerals
- Various sugars
- Fruit
- Nuts
- Seeds
- Algae
- Molluscs and crustaceans
- Insects
- Bakery products
Analytical Constituents
Regulation 767/2009 requires that under “Analytical Constituents” of both complete and complementary feeds the typical analysis of nutrients must be declared. In Europe, the typical (proximate) analysis has to be declared. This is the typical nutrient level obtained by considering the results of analysis of several samples. In other words, the typical analysis gives the nutrient levels found in the actual food. The declaration of the following nutrients is mandatory for pet foods for dogs and cats:
- Crude Protein
- Crude oils and fats
- Crude fibre
- Crude ash*
- Moisture if >14%
The declaration of nutrients such as calcium, phosphorus, sodium, magnesium and other nutrients is optional. However, all nutrients must be declared to which a manufacturer draws attention either as a picture, icon or in words e.g., stating that the food is “high or rich in nutrient A”.
In addition for pet food there is a derogation that permits "crude protein" to be replaced by "protein" and "crude oils and fats" to be replaced by “fat content".
- Ash is the term used to define the inorganic material left after organic material has been burnt. It is also permitted to call “ash”, “incinerated residue” or “inorganic matter”.
Additives
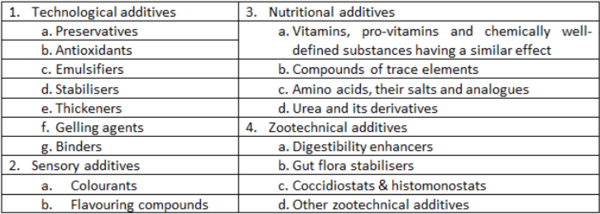
Within the EU, Regulation (EC) No 1831/2003 on additives for use in animal nutrition provides for 4 categories of additives (1-4), each of which contain functional groups (Table 2). More categories may be added over time. Vitamins and trace elements are considered additives and, therefore, are not listed under composition.
Which Additives Must Be Declared on the Label?
According to Regulation 767/2009, the following additives have to be declared if added by the manufacturer:
- Additives where a maximum content is set for any kind of target species (see Register of additives for links to authorising legislation). This means that, for example, if an additive has a maximum inclusion level for calves, it has to be declared also on the label of cat foods, if added. This is because pet foods and livestock feed are regulated by the same legislation.
- Additives belonging to the categories
- Zootechnical additives
- Coccidiostats & histomonostats
- Additives belonging to the functional group ‘urea & derivatives’
- Any other additive if its presence is emphasized on the label, whether in words, picture or graphic.
How Additives Must Be Declared:
The specific name of the additive as defined in the relevant legal act authorising the additive and/or its identification number* must be declared in a list preceded by the name of the functional group or category to which they belong (Table 2). In addition to the name, the added amount of the additive must be declared. The list of additives has to be preceded by the heading ‘additives’.
- N.B. Since the beginning of the reauthorisation of feed additives under Regulation 1831/2003 as amended, E numbers are being replaced with new identification numbers.
Derogations
For pet food, additives of the functional groups “preservatives, antioxidants and colorants” with a maximum legal level, only the respective functional group can be declared. In this case, the name, identification number and the functional group of the feed additive, which are not declared, shall be disclosed to the purchaser on his request.
Particular Nutritional Purposes (PARNUT)
PARNUT = Feeding stuffs for PARticular NUTritional purposes
Articles 9 & 10 of Regulation 767/2009 provide the basis for feeding stuffs intended for particular nutritional purposes, in other words ‘dietetic (pet) foods’. Feed intended for particular nutritional purposes may only be marketed as such if the intended use (PARNUT indication) is included in the list of intended uses published in Directive 2008/38/EC, which establishes a list of intended uses of animal feeding stuffs for particular nutritional purposes.
What are Particular Nutritional Purposes?
A Particular Nutritional Purpose is intended to meet the specific nutritional needs of animals whose process of assimilation, absorption or metabolism is, or could be, temporarily or irreversibly impaired and who can, therefore, benefit from a feed that is adapted to the specific needs generated by their condition. The objective of the PARNUT legislation is to fill the gap between the legislation for feed for healthy animals, for which health claims are not permitted, and the legislation on medicated feed.
The term ‘dietetic pet food’ (and its official translations) is the only term to be used to indicate that a product falls under this legislation. These label declarations apply in addition to rules already in place for regular pet food products.
Directive 2008/38/EC
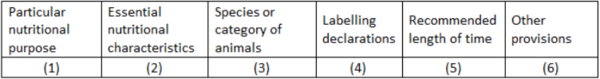
Directive 2008/38/EC lists all permitted PARNUTS (1), together with the essential characteristics (2) needed to meet the requirements for the specific PARNUT, the species (3) for which it is indicated, the additional mandatory labelling requirements (4) and other provisions where necessary (6) (Table 3). Consequently, a number of additional statements appear on the labels of dietetic pet foods.
Since the legislator tends to consider most PARNUTs as being ‘temporary situations’, they make it mandatory to publish on the labels a defined length of time for use (Table 3, column 5). However, not all PARNUT indications are temporary, e.g., diabetes mellitus type 1 and chronic kidney disease are irreversible and need lifelong dietary management. This issue has been resolved by stating in column 5 ‘initially up to xx weeks or months’. In the same time this statement should stimulate the pet owner to visit the treating veterinarian for regular control check-ups.
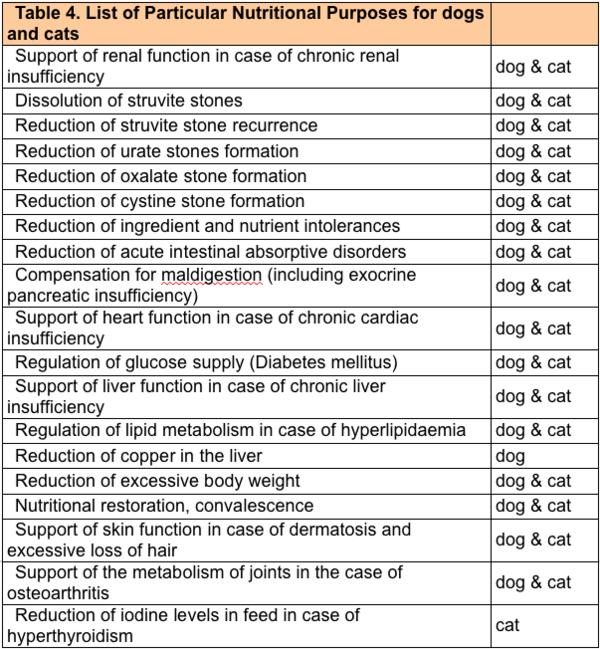
Feeds intended for particular nutritional purposes shall be marketed only if their intended uses are included in Part B of Annex I to this Directive and if they fulfil the other provisions laid down in that Part of Annex I (columns 2 to 6). The PARNUTs currently approved for dogs and cats are listed in table 5.
How is it Decided Whether a Condition is a Nutritional Purpose and is Included in Directive 2008/38/EC?
The Commission may update the list of intended uses set out in Directive 2008/38/EC by adding an intended use, withdrawing an intended use or by adding, removing or changing the conditions associated with a particular intended use. In order to add a new nutritional purpose or modify an existing PARNUT, a dossier must be introduced to the EU Commission, showing sufficient scientific data to support that 1) the indication matches the definition of PARNUT and 2) a diet with the proposed essential characteristics has a beneficial effect on the specific condition.
Claims
Regulation 767/2009 states that the labelling and the presentation of feed shall not mislead the user, in particular:
a. As to the intended use or characteristics of the feed, in particular, the nature, method of manufacture or production, properties, etc. (article 11).
b. By attributing to the feed effects or characteristics that it does not possess or by suggesting that it possesses special characteristics when in fact all similar feeds possess such characteristics (article 11);
c. By claiming that it will prevent, treat or cure a disease (article 13). Such claims are considered medicinal and imply that the product is a medicinal drug and should comply with all regulations governing veterinary medicines.
d. By claiming that it has particular nutritional purpose (PARNUT), but is not included in the list of permitted PARNUTS published in Directive 2008/38/EC (table 5) (article 13).
Any claim referring to the product must be substantiated, not confuse or mislead purchasers and not denigrate other pet foods.
Regulation 767/2009 provides for industry to develop Codes of Good Practice to encourage both self-regulation in areas where regulation is top-level or non-existent, and to help promote a “level playing-field” approach with the EU. To that end, FEDIAF have developed a Code of Good Labelling Practice for Pet Food Labelling that provides more detailed guidance on claims and claims substantiation, including the definition of specific terms such as “fresh”, “natural” etc. The Code also contains a layman’s section where the key elements of pet food labelling, and the legal requirements behind them, are explained. The current version of the Code was recognised by the EU Commission in 2011.
Useful Websites
General:
- European Animal Feed Safety
- Specific Legislation
- FEDIAF code of good labelling practice for pet food
USA
Pet foods distributed in more than one state of the USA are subject to a host of labelling requirements covering aspects such as product names, ingredient lists, nutrient content guarantees, and nutritional adequacy statements (Dzanis ’08). An example of such a label is shown in Figure XX. In addition to meeting the federal labelling requirements, animal feed products are also subject to individual state laws. In the United States, the majority of states have adopted and enforce the AAFCO Model Regulations for Pet Food and Specialty Pet Food. AAFCO is the official source of information on pet food labelling, ingredient definitions, official terms and standardized feed testing methodology. The approach to labelling in Canada is similar to the USA. However, Canada does not regulate pet food labels, except under the broader Consumer Packaging and Labelling Act of the Competition Bureau which covers all retail packages, including requirements for dual language (English/French) declarations. There is a “Guide for the Labelling and Advertising of Pet Foods” which generally follows AAFCO, although it has no legal force. Most manufacturers in Canada voluntarily choose to follow AAFCO.
Label Design
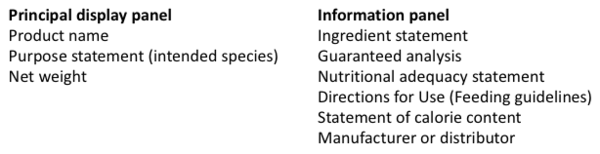
Similar to Europe, a pet food label is divided into two main parts: 1) the principal display panel and 2) the information panel.
Table 6. Elements legally required on pet food labels in the United States of America and Canada.
Optional declarations on the – principal display panel (PDP) are the brand name, the manufacturer’s name, and claims in words, pictures or graphics (such as the product vignette, bursts and flags).
Principal Display Panel
The principal display panel is the part of a label that is most likely to be displayed to customers at retail facilities. It is a primary means of attracting the consumer’s attention to a product and is usually used to communicate important information about the product.
Product Identity
Beside the product name, which is legally required, the product identity can also include the manufacturer’s name, a brand name or both. It is not mandatory to include the manufacturer’s name as part of the product identity on the principal display panel, but the name and address of the manufacturer (or distributor, when the product is manufactured for another party) must appear somewhere on the label. The product name often contains some description of the food and is subject to AAFCO regulations about composition of ingredients such as the percentage rules.
Percentage Rules
The percentage rules determine the terminology that can be used to describe a product depending on the inclusion level of an ingredient. For instance, the terms “lamb dinner”, “lamb recipe” or a similar designation requires that lamb (not including by-products or derivatives such
as lamb meal or lamb liver) make up at least 25 % of total weight of all ingredients. For wet foods the water added during processing may be excluded from the percentage calculation, but never be less than 10% of the total product (AAFCO). An ingredient less than 25% but that comprises at least 3% of the formulation (exclusive of water for processing) may be indicated in the name or elsewhere on the label only in conjunction with words such as "with" or "contains," and must fall below maximum type size requirements to avoid overemphasis of minor ingredients.
Intended Species
The words “dog food” or “cat food” or similar terms must appear conspicuously on the principal display panel of pet foods. It must identify the animal for which the product is intended, so that it is clear that the product is not intended for humans.
Net Weight
The declaration of the net weight (or volume, in the case of liquid products) on the principal display panel is mandatory under FDA regulations. Net content declarations must be displayed in conspicuous and easily legible print, within the bottom 30% of the principal display panel in lines generally parallel to the base of the package. There are type size requirements for the net weight statement based on size of the Principal Display Panel.
Information Panel
Ingredient Statement
Unlike the EU, each ingredients in the pet food must be listed in descending order by weight (i.e., ingredient categories are not allowed). AAFCO has established the name and definition of a wide variety of ingredients. The ingredient names on the label must conform to the AAFCO name (e.g., poultry by-product meal, corn gluten meal, powdered cellulose), or when an applicable AAFCO name is lacking, be declared by their common or usual names, such as beef or chicken. Collective terms, such as animal protein products or ingredient brand names are not allowed on pet food labels. Contrary to the EU, in the USA trace elements and vitamins are considered ingredients and are listed in the ingredient statement. In the same way, additives such as antioxidants, preservatives, humectants, colouring agents, flavours, etc. must be listed in the ingredient statement.
Guaranteed Analysis
Unlike pet food labels in the EU, where the typical analysis of nutrients has to be declared, in the United States minimum / maximum guarantees are declared on pet food labels (Table 7). It is important to recognize that these percentages generally indicate the “worst case” levels for these nutrients in the food. Although the guarantees do not reflect the exact or typical amounts of these nutrients, this method of declaration provides a sound means of verification and enforcement by regulators. The maximum moisture content in pet foods cannot exceed 78%, except in products labelled as a stew, gravy, broth, juice, milk replacer or similar terms.
In addition to the above, a maximum percentage crude fat guarantee is required on any dog or cat food label bearing a "low fat" "lean," "less fat" or similar claim. Although a maximum percentage ash* guarantee is not legally required in the United States, many pet food manufacturers include one on the labels of their foods. Guarantees for other nutrients may also be declared, the declaration of a nutrient is not mandatory unless its presence is highlighted elsewhere on the label, for example, "with taurine" would require a minimum percentage taurine guarantee. If these additional guarantees are for essential nutrients (e.g., taurine in cat foods),, they must be declared following the same terms and units of measure outlined in the AAFCO Nutrient Profiles. If the nutrients are not essential (e.g., taurine in dog foods), a disclaimer stating so must be asterisked to each nonessential nutrient.
- Ash is the term used to define the inorganic material left after organic material has been burnt.
Feeding Guidelines
All "complete and balanced" pet foods must provide quantitative feeding directions for each life stage indicated in the nutritional adequacy statement, unless a more restricted life stage designation is more prominently indicated elsewhere. The feeding directions must at minimum state the amount of feed (e.g. cups) per given body weight dog or cat and the frequency of feeding, although especially on larger packaging, more detailed directions, often in tabular format, are typically provided.
Statement of Calorie Content
Almost all dog and cat foods must carry a calorie content statement. This requirement includes all complete and balanced foods and all snacks, treats and non-exempt chew products. The statement is separate from the guaranteed analysis and appears under the heading ‘calorie content’. The declared energy is the metabolizable energy and must be expressed as kilocalories per kilogram (kcal/kg) of product on an ‘as fed’ basis, as well as in kilocalories per familiar household measure (e.g., kcal/cup, kcal/can, per piece). Rawhides, bones, ears and other chews that are exempt from AAFCO registration and labelling requirements are not required to display a calorie content statement. The method of determination (calculated vs. feeding trials) must also be clearly identified in the statement. The method for determining calories by digestibility trials is described in protocols in the AAFCO Official Publication.
The calculation method, which is also described in the AAFCO Official Publication & AAFCO’s "Business of Pet Food" website (www.petfood.aafco.org), is based on the average analysis (not the guaranteed analysis) and uses the modified Atwater factors: kcal ME/kg = (% crude protein x 3.5 + % NFE x 3.5 + % crude fat x 8.5) x 10, where NFE (the non-structural carbohydrate fraction) is determined by subtracting the combined measured fractions (crude protein, crude fat, crude fiber, moisture and ash) from 100.
Name and Address
The name and address of the guarantor, who may be the manufacturer, importer, or distributor must be stated on the label, on the information panel. Phrases as ‘distributed by...’, ‘manufactured for...’ or ‘imported by...’may indicate that a company other than the one selling the product has manufactured the pet food. This is a common practice with private label pet foods. Products that are imported must indicate the country of manufacture alongside the Guarantor Statement.
Complete and Balanced - Nutritional Adequacy Statement
In the USA, all pet food labels, with the exception of products clearly labelled as treats and snacks, or supplements must contain a statement of nutritional adequacy and manufacturers must indicate the method and life stage that was used to substantiate this claim (validation of nutritional adequacy). AAFCO regulations allow three methods to substantiate such claims:
- Based on product formulation
- Based on feeding trials, and
- Based on "product family" criteria .
In 1990 and 1991, AAFCO convened the Canine Nutrition Expert (CNE) and Feline Nutrition Expert (FNE) subcommittees to establish practical nutrient profiles (minimums and maximums) for cat and dog foods based on commonly used ingredients. The profiles were last updated in 2016. The reports of the CNE and FNE subcommittees (and subsequent revisions) form the basis for the nutrient profiles that are published in the annual edition of the AAFCO Official Publication. The formulation method allows a manufacturer to claim ‘complete and balanced’ for a given life stage or life stages when the product is formulated to meet the AAFCO Dog or Cat Food Nutrient Profiles for that life stage(s). These nutrient profiles cover two categories:
- Growth and reproduction and
- Adult maintenance (AAFCO 2015).
For example, a pet food meeting the AAFCO nutritional profile of adult dogs can bear the following statement: ‘(Name of product) is ‘formulated to meet the nutritional levels established by the AAFCO Dog Food Nutrient Profiles for maintenance of adult dogs.’ The most recent revision of the AAFCO Dog Food Nutrient Profiles include a lower maximum calcium level in foods that MAY be fed to large-breed puppies. All products indicated "for growth" or "for all life stages" and that contain no more than 1.8% Ca on a dry matter basis must include the clause "including growth of large size dogs (70 lbs or more as an adult)." Dog foods exceeding that amount (but still in conformance with the maximum calcium allowance for all dog foods) must state "except for growth of...).
The second method requires that the manufacturer performs an AAFCO-protocol feeding trial using the food as the sole source of nutrition. The feeding trial method can result in a nutritional adequacy claim for one or more of the following categories:
- Gestation and lactation,
- Growth,
- Adult maintenance and
- Complete for all life stages.
AAFCO has published minimum testing protocols for adult maintenance, growth and gestation/lactation. A food that successfully completes a gestation/lactation trial, followed by a growth trial using the puppies/kittens from the gestation/lactation trial, can also make a claim for all life stages. The required terminology for labels of pet foods that have passed these tests is: ‘Animal feeding tests using AAFCO procedures show that (brand YY) provides complete and balanced nutrition for (lifestage xx)’. The adult maintenance protocol uses a minimum of eight animals that are fed the food as the sole source of nutrition for six months, during which the animals are evaluated for any change in body weight and other clinical signs of nutritional deficiency or excess and blood analyses are performed. Growth protocols include feeding the food for a minimum of 10 weeks during the most critical growth phase to maximize the ability to detect nutritional problems with the food. Evaluation of feeding trials to support nutritional adequacy for pregnant or nursing dogs and cats also looks at additional parameters, such as litter size and puppy/kitten weight at the end of the trial.
The third method (product family criteria) requires the testing of a representative lead member through the appropriate feeding trials. To be designated a member of the product family, foods must be the same processing type and in the same moisture category, be intended for the same or more restrictive life stages, be similar in energy density (±7.5% kcal/kg on a dry matter basis), and meet other nutritional criteria compared to the lead product. Provided that the calorie content of both products are determined by digestibility trials, product family members are allowed to bear the same "animal feed tests..." statement as found on the tested product.
Products substantiated by the latter two methods may be subject to re-substantiation upon any changes to the formulation or after 5 years on the market. The label for pet foods that fail to meet AAFCO requirements by any of the three standard methods (feeding trial, product family or formulation) and is not prominently identified as a snack, treat, or supplement must bear the statement: This product is intended for intermittent or supplemental feeding only.
Veterinary therapeutic foods, which are intended for use by or under the supervision or direction of a veterinarian, must be substantiated for nutritional adequacy by one of the above methods or its label must bear the "intermittent or supplemental" statement as required for all dog and cat foods. However, labels for these foods may bear the statement ‘use only as directed by your veterinarian’ in lieu of feeding directions. Technically, veterinary therapeutic foods are drugs as defined by FFDCA, and have not undergone the approval process as required by the law for drugs. However, FDA exercises enforcement discretion in not taking action against these products provided they meet FDA's guidelines.
Batch Information
Information such as batch code and date of manufacture are also frequently found on pet food labels, and companies often use the ‘best before date’ or something similar to indicate the freshness date of the product. A method of traceability is also required and this information can be used to meet this need. Because of the need to continuously revise the code during production, this information is typically stamped on the label or elsewhere on the container (e.g., the bottom of the can), not necessarily in conjunction with either the principal display panel or the information panel.