Neurons - Anatomy & Physiology
Introduction
Nerves allow electrical impulses to propagate along their elongated cell extensions and facilitate the transfer of information throughout the body. Neural tissue is found within the central nervous system (CNS) and the peripheral nervous system (PNS) and the composition and constituent parts of neurones and their surrounding cells differ only slightly.
CNS Neurons
Neural tissue found in the CNS is composed of gray and white matter. The gray matter includes nerve cell bodies and some short branches from these cell bodies. The white matter is composed of the long extensions of the nerve, the nerve fibres.
Within the central nervous system there are two major cell types; neurons which are the "functional" cells of the central nervous system and glial cells which play a supporting role within the CNS. Within the CNS there are also a number of other cells that play a supportive role to the neurone and these include astrocytes, oligodendrocytes, microglial cells, ependymal cells and choroid plexus epithelial cells.
PNS Neurons
The PNS contains a series of paired nerves that extend from the spinal nerves originating from the spinal cord and cranial nerves that originate from the brain stem. Neurones found in the PNS are similar to those found in the CNS aside from a few subtle differences. Supportive cells that surround the nerves in the PNS are called Schwann cells (rather than Glial cells). The main neurotransmitter found in the PNS is acetylcholine.
Neurons
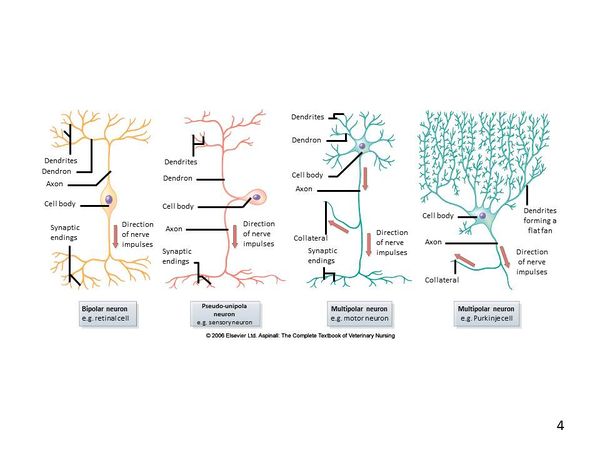
A typical neuron can be exemplified by a motor neuron in which the cell body of the nerve is located within the gray matter of the spinal cord and the nerve fibre, or axon, extends to the muscle. Nerve axons can be very long permitting electrical impulses to be sent over long distances throughout the body. The information below specifically regarding neurons is interchangeable between the CNS and PNS and therefore links have been provided where appropriate on this page to the PNS Structure.
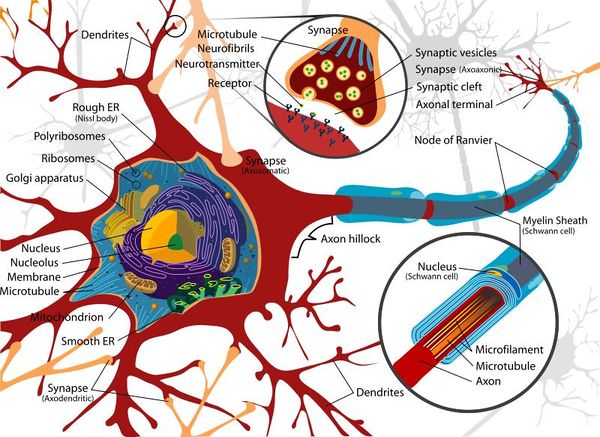
Basic Nerve Structure
The basic structure of a nerve is that of a cell body (soma), which has a single long nerve fibre (axon), attached at one end to the cell body and at the other end to another nerve cell body or to a structure requiring nerve impulses such as skeletal muscle. The interface between two nerves or a nerve and another structure is called the nerve synapse. The cell body of the nerve itself also contains numerous dendrites which increase its surface area enabling other nerve axons to connect with the cell body. The cell body usually has connections with many other axons from other nerve cells with many synapses on the cell body. The axon of the nerve cell is usually surrounded by some form of insulating protection, or myelination. This protective layer is called the myelin sheath.
Soma (Cell Body)
The soma, or cell body, is the central part of the neuron and contains the nucleus of the cell, the rough and smooth endoplasmic reticulum, ribosomes and golgi apparatus. Similar processes that would be undertaken by any cell occur within the soma and due to the organelles it contains, the soma is where most protein synthesis occurs.
Dendrites
The neuron's dendrites are effectively branching extensions of the cell body. Collectively, these branching structures of the dendrites are known as the "dendritic tree". The dendritic tree is the site where input to the neuron occurs via synapses with axons from other nerve cells. In most cases nerve impulses travel from other nerve cells to the cell body and are then conducted along the nerve cell's own axon to other cell bodies. However, dendrites themselves are unable to propagate nerve impulses in the manner of axons as dendrites are unable to secrete neurotransmitters. Similarly axons do not possess the chemoreceptors that are found within the dendrites and are therefore unable to receive nerve impulses. Nerve impulses are therefore conducted in one direction only.
Axon (Nerve Fibre)
The axon is a very fine projection that can measure up to thousands of times the diameter of the soma in length. The axon carries nerve signals away from the soma. The structure and function of the axon is very similar between the CNS and the PNS.
Axon has been covered in detail within the PNS Structure and Anatomy page.
Glial Cells
Glial cells make up approximately 50% of the volume of the nervous system and the number of glial cells out-numbers the nerve cells by a factor of ten to one. The primary function of glial cell is to provide support to the neuronal cells. There are several different types of glial-style cell; in the PNS these are referred to as Schwann cells and whilst in the CNS they are referred to as Oligodendrocytes.
Glial cells form a protective networked layer around the neuron within the CNS and also help to maintain the fluid content of the tissue surrounding the nerve. This protective layer is referred to as the myelin sheath and axons that are wrapped within this myelin sheath are able to conduct nerve impulses at higher speeds than those that are not wrapped. During foetal development the glial cells wrap around axons numerous times and as the glial cell matures it loses the majority of its cytoplasm. The remaining cellular structure consists of many layers of tightly packed lipid membranes around the axon.
Periodically along the surface of the wrapped glial cell there are gaps approximately every 1-2mm. Each gap allows the environment external to the myelin sheath to be exposed to the axon for approximately 1-2υm. These gaps in the sheath are called the Nodes of Ranvier and are important in the conduction of impulses along the axon.
Schwann Cell
Details regarding the Schwann cell have been covered within the [PNS Structure - Anatomy & Physiology#Schwann Cell|PNS Structure and Anatomy]].
Oligodendrocytes
The term "oligodendrocyte" literally means a "cell with many branches". Oligodendrocyte precursors are found throughout the CNS and these precursors are post-mitotic, meaning oligodendrocytes can be replaced. Oligodendrocytes can myelinate up to 40 axons at a time. The myelin sheath formed by oligodendrocytes has numerous functions including; decreasing any ion leakage from the axon lowering the capacitance of the cell membrane and increasing impulse speed along the axon by allowing saltatory conduction of action potentials between the nodes of Ranvier.
Nerve Impulse Propagation
Nerves are able to create, amplify and propagate electrical impulses that run along their axon. These nerve impulses are a form of action potential that is carried by ions. The nerve impulse is in effect an electrical difference between the inside and outside of the axon and is caused by ion movements across the membrane. Nerve impulses can occur from a number of sources including sensory cells, action potentials from other connected nerves or spontaneous depolarisation of the nerve cell membrane.
Unmyelinated Axons
Conduction of an action potential along an unmyelinated axon, i.e. an axon not covered by some form of glial cell, is a much slower form of nerve impulse propagation than that of a myelinated axon.
At a given moment when the action-potential threshold is reached voltage-gated sodium channels within the membrane of the axon will open resulting in an influx of ions following their electro-chemical gradient. This Na+ influx causes the axon to accumulate a positive charge and results in cellular depolarisation. The extracellular area of the axon therefore looses its positive charge, becoming more negative resulting in a current of positive charge that flows through the tissue towards the axon. The membrane of the axon is not a perfect insulator and at some regions on the axon, particularly within the area of the axon in front of the action potential, the voltage-dependant ion channels have not activated yet. This means that some of the positive charge is able to flow out of the axon membrane in these regions and this positive charge outflow is mainly via potassium. This outward leakage of potassium results in the current within the axon only being able to travel a short distance before the nerve impulse decays. However the effect of the current locally on the voltage-gated channels means that the nerve impulse is able to open voltage-gated channels within its immediate vicinity and this is enough for the signal to propagate along the nerve.
The axon membrane at rest has a slightly negative charge and the local current of positive charge during an action potential reduces this negative charge. This process is called depolarisation and when this has progressed sufficiently, it results in the membrane potential reaching threshold level allowing the voltage-gated sodium channels to open. These channels only stay open for approximately 0.5ms and when they close the membrane is depolarised for a further 0.1-0.5ms. These voltage-gated channels are unable to open again for a short period post depolarisation. This is called the refractory period.
The internal electrical resistance within the axon is the determining factor regarding how fast the local positive current impulse can pass through. The less the internal resistance, the higher the conduction velocity of the nerve impulses due to the locally positive current being able to depolarise the membrane to threshold level over a greater distance. This internal resistance decreases as a function of axon diameter an therefore thicker axons are able to conduct nerve impulses more rapidly.
Myelinated Axons
The addition of the myelin sheath to nerve axons greatly enhances the speed with which they are able to conduct nerve impulses. Unmyelinated nerves are able to conduct up to speeds to 25m/s whilst myelinated nerves are capable of up to 100m/s. For an unmyelinated nerve to conduct impulses at the same rate, it would have to have a diameter of approximately 100 times that of the myelinated nerve.
As with unmyelinated axons, an area of locally positive charge flows through the cytosol from the activated area within the axon. The major difference with myelinated nerves is that where the current effectively leaks away in unmyelinated nerves, here the current is only able to leak away through nodes of Ranvier (see above). Therefore even a weak current is able to depolarise the axon membrane to the threshold level. There is a small loss of current but as these nodes are relatively small the local current is able to travel much further in myelinated axons resulting in a much higher conduction velocity.
The influx of sodium ions via voltage-gated channels is only possible at these nodes and therefore the nerve impulse is able to effectively 'jump' from node to node. This type of impulse propagation is called saltatory conduction. Myelination of axons in mammals means that the nervous system can sustain a large number of high velocity axons within a relatively small space.
Synapses
The synapses found at the end of axons are fundamental to the functioning of the nervous system as they facilitate communication between nerves and provide an interconnected network for many of the complex processes required by organisms. Synapses are required as the lipid bi-layer of the cell membrane has a relatively large electrical resistance making electrical impulse propagation directly between cells difficult.
The most common form of nerve synapse is the chemical synapse which utilises neurotransmitters. When a nerve impulse reaches a synapse, neurotransmitters are released by the presynaptic terminal of the synapse and these transmitters diffuse to the membrane of the post-synaptic membrane where they bind to receptors. These receptors cause an inhibition or excitement in that nerve resulting in either blocking further electrical impulses or the further propagation of a signal.
Neuromuscular Synapses
These chemical synapses provide connection between nerves and skeletal muscle cells. These synapses are most commonly found residing within groups of muscle cells where each neuron is in contact with several muscle cells but each muscle cell is only ever connected to one neuron. The nerve axon branches out prior to the muscle cells allowing multiple synapses with muscle cells from a single nerve axon. A synaptic cleft of approximately 30-50nm is found between the nerve synapse and the muscle cell.
The nerve terminal membrane or pre-synaptic membrane contains numerous vesicles that contain the neurotransmitter, in this case most commonly acetylecholine (ACh). Once released from the vesicles the ACh diffuses across the synaptic cleft and binds to receptors in the muscle cell membrane. This binding causes ligand-gated ion channels to open which are permeable to both Na+ and K+. The movement of potassium is relatively small due to there being only a small electrochemical gradient between the extra and intracellular environment. However there is a large influx of sodium into the cell and consequently this causes depolarisation in the muscle cell thus propagating the impulse into mechanical movement. Within the neuromuscular junction, the release of vesicles is facilitated by an influx of calcium into the pre-synaptic nerve just prior to exocytosis. The calcium enters the pre-synaptic nerve via voltage-gated Ca2+ channels. There are several mechanisms that reduce the intracellular concentration of calcium once vesicles begin to be released to ensure that the neurotransmitter release is brief to prevent hyperpolarisation.
The particular neurotransmitter ACh is heavily recycled within the synaptic cleft via endocytosis and over time the levels of endocytosis and exocytosis balance one-another resulting in a stable pre-synpatic membrane. Depolarisation within the muscle cell will last as long as the ACh is present in sufficient quantities within the synaptic cleft. In reality this only lasts a few milliseconds as the synaptic cleft also contains the enzyme acetylcholinesterase which hydrolyses the ACh into acetate and choline. Due to this mechanisms, the calcium restriction and the endocytosis, only one action potential is generated within the muscle fibre.
Inter-neuron Synapses
The interaction between nerves within the synapse is fairly similar to that within the neuromuscular junction but there are several key differences. Firstly the receiving neuron will be receiving information from multiple other nerves rather than just one nerve as per a muscle cell. Secondly there are many more types of neurotransmitters utilised by inter-neuron synapses than just ACh. There are also a wider range of types of synapses between neurons that include both excitatory which will propagate a nerve impulse, but also inhibitory which will prevent another synapse on the same nerve propagating a nerve impulse.
Excitatory synapses will lead to depolarisation of the target neuron and if this depolarisation reaches the threshold level, an impulse will be propagated. This depolarisation is referred to as excitatory post-synaptic potential (EPSP) as it brings the membrane potential of the target cell closer to the threshold for an action potential. If several excitatory synapses on the same nerve excite simultaneously, the target cell will receive the sum of all these individual synaptic potentials. Therefore multiple e
active excitatory synapses are more likely to cause a threshold depolarisation.
Inhibitory synapses between neurons also have many synapses from other nerves connecting to a single nerve providing a network of connections. However, the neurotransmitter molecules within an inhibitory synapse usually open ligand-gated channels for chloride-ions and potassium ions. K+ will then flow out of the cell whilst Cl- flows into the cell resulting in the cytosol of the cell becoming more negative. This adjustment in membrane potential is called a inhibitory postsynaptic potential (IPSP) as it takes the membrane potential of the cell further from the threshold level required to generate an action potential.
Astrocytes
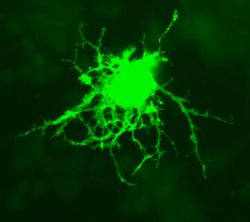
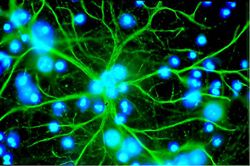
Astrocytes, or astroglia, are star-shaped glial cells within the brain which have many processes that envelope synapses made between neurons. Astrocytes have several functions including the biochemical support of the endothelial cells in forming the blood-brain barrier, provision of nutrients to nervous tissues and to form scar tissue during repair of the brain. The fluorescent image of astrocytes on the right is possible because astrocytes express glial fibrillary acidic protein (GFAP) which facilitates their identification. The following paragraphs provide a more detailed overview of the many functions of the astrocyte within the brain.
Another key role of astrocytes within the brain where they play an important structural role as well as performing the roles described above. Astrocytes are involved in the physical structuring of the brain and the interplay between astrocytes and mesenchymal cells is important in sculpting sulci in development. Astrocytes also delineate the CNS from the PNS by creating the CNS-PNS interface. The cells cover the surface of the brain and where this occurs the astrocytes' surface is coated by a basal lamina.
Astrocyte Functions
Where astocytes are involved in repair within the brain they take over from fibroblasts to play an important role in abscess formation, as it is difficult to induce fibrosis within the CNS. It is due to this role that astrocytes are the only differentiated CNS cell that retains the ability to divide.
Within the metabolic role that astrocytes play within the brain, it has been found that they may control glucose levels and provide lactate for neurone for energy production. The lactate is believed to be produced via the lactate shuttle mechanism. Astrocytes also have high levels of antioxidants, such as glutathione peroxidase which protects the neurons from damage by reactive oxygen species.
Perhaps one of the most important roles that is undertaken by astrocytes is that of the blood brain barrier (BBB). It was originally thought that the astrocyte "end-feet" that encircle adjacent endothelial cells within the BBB aided in the maintenance of the blood-brain barrier. However, the tight junctions and basal lamina of the endothelial cells are now considered to play a more substantial role in maintaining the barrier and within this astrocytes play a key role.
Some classifications of neurotransmitters, namely small-molecule neurotransmitters are reabsorbed and subsequently recycled. Astrocytes represent an element of the system that enables this neurotransmitter re-uptake. Astrocytes are able to effectively isolate synapses and control their potassium levels as well as expressing several types of transporter for the numerous types of neurotransmitters, including glutamate, ATP and GABA. Glutamate is particularly well taken up, and converted to glutamine using glutamine synthase. The role played in controlling potassium ion concentrations within the extracellular spaces involves astrocytes releasing K+ when neurons are active, increasing the local extracellular K+ concentration. Astrocytes have a high density of K+ channels, allowing them to rapidly clear the excess accumulation in the extracellular space. If this function fails extracellular K+ concentration rises leading to neuronal depolarisation, and may be a cause of epilepsy.
Astrocytes may serve as intermediaries in the neuronal regulation of blood flow by promoting the myelinating activity of oligodendrocytes. Neuronal electrical activity releases ATP, which has been shown to stimulate myelin formation. This stimulation occurs via astrocytes, which secrete LIM in response to the ATP. LIM is a regulatory protein that promotes the myelinating activity of oligodendrocytes.
Astrocytes may play a crucial rule in controlling inflammation in the CNS by secreting chemokines. Astrocytes have also been shown to be able to restrict the entry of inflammatory cells to the CNS. When astrocytes are lost or destroyed, the surrounding neurons become very vulnerable in the face of inflammation.
Microglial Cells
Microglia are the CNS version of a mononuclear phagocyte system as the normal phagocytic white blood cells are unable to cross the blood-brain barrier. Microglial cells are derived from bone marrow and are first seen in the brain just prior to the vascularistion stage of brain development. There are two populations of microglial cells; extrinsic cells which are located perivascularly and in the meninges, and intrinsic cells which are located within the substance of the neural tissue.
The microglia effectively act as macrophages where they rapidly respond to any type of injury. However, microglia differ from other representatives of mononuclear phagocyte system by their low expression of MHC-2. When undertaking a phagocytic response, MHC-2 can be easily up-regulated. Each type of microglia has a different turnover rate; perivascular and meningeal microglia turnover fairly rapidly whilst intrinsic microglia have a very slow turnover.
Ependymal Cells
Ependymal cells make up the epithelial membrane which is the lining of the ventricular system within the brain and spinal cord. Ependymal cells are are involved in the production of cerebrospinal fluid. The cells line the CSF-filled ventricles in the brain and the central canal of the spinal cord, and form part of the choroid plexus. The apical surfaces of ependymal cells are covered with
a layer of cilia and microvilli.
Within the ventricles, modified ependymal cells and capillaries form a system called the choroid plexus which produces CSF. The ependymal cells beat thier cilia to circulate CSF around the central nervous system and the microvilli absorb CSF.
Endothelial Cells and the Blood Brain Barrier
The walls of capillaries throughout the body and within the CNS are formed by endothelial cells, however, within the CNS the capillary endothelial cells are very tightly packed. This density of cells restricts the passage of substances out of the bloodstream much more than endothelial cells in capillaries elsewhere in the body. This is the basis of the blood brain barrier.
Neuronal Transport Mechanisms
Neurons are often quite large when compared to other cell types within the body and therefore the transport of molecules, organelles and other elements important to the cell becomes more difficult and equally more important. Neurons have developed a number of mechanisms to transport material throughout the cell.
Anterograde Transport
Anterograde transport facilitates the movement of substances in the direction of the cell body to the axon. This type of neuronal transport has been covered in detail within the PNS Structure and Anatomy page, please click here.
Retrograde Transport
Retrograde transport is the opposite of anterograde in that it returns materials from the axon terminal to the cell body, either for degradation or restoration and reuse. This type of neuronal transport has been covered in detail within the PNS Structure and Anatomy page, please click here.
Neuron Blood Supply
The blood supply to nerves is called the vasa nervorum. The blood supply to neurons has been covered in detail within the PNS Structure and Anatomy, please click here.
Error in widget FBRecommend: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a5eae25eece0_76258029 Error in widget google+: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a5eae263d4c6_68941225 Error in widget TwitterTweet: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a5eae2693890_07254998
|
| WikiVet® Introduction - Help WikiVet - Report a Problem |