Difference between revisions of "Dirofilaria immitis"
Fiorecastro (talk | contribs) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {{OpenPagesTop}} | |
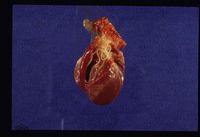
[[Image:Dirofilaria immitus.jpg|thumb|right|250px|''Dirofilaria immitis'' - Courtesy of the Laboratory of Parasitology, University of Pennsylvania School of Veterinary Medicine]] | [[Image:Dirofilaria immitus.jpg|thumb|right|250px|''Dirofilaria immitis'' - Courtesy of the Laboratory of Parasitology, University of Pennsylvania School of Veterinary Medicine]] | ||
| Line 19: | Line 19: | ||
==Pathogenesis== | ==Pathogenesis== | ||
| − | Heartworm disease primarily affects the cardiopulmonary system and the severity and extent of lesions depends on several factors. These include the number and location of adult worms<sup>1, 2</sup>, the duration of infection, and the level of activity of the host<sup>1</sup>. Parasites in the pulmonary arteries cause mechanical irritation, leading to endothelial damage, proliferation of the intima and perivascular cuffing with inflammatory cells. This results in narrowing and occlusion of the vessels which in turn causes pulmonary hypertension. A combination of pulmonary hypertension and inflammatory mediators can lead to an increase in the permeability of pulmonary vessels, giving periarterial oedema and intersitial and alveolar infiltrates. Eventually, irreversible interstitial fibrosis arises. | + | Heartworm disease primarily affects the cardiopulmonary system and the severity and extent of lesions depends on several factors. These include the number and location of adult worms<sup>1, 2</sup>, the duration of infection, and the level of activity of the host<sup>1</sup>. Parasites in the pulmonary arteries cause mechanical irritation, leading to endothelial damage, proliferation of the intima and perivascular cuffing with inflammatory cells. This results in narrowing and occlusion of the vessels which in turn causes pulmonary hypertension. A combination of pulmonary hypertension and inflammatory mediators can lead to in an increase in the permeability of pulmonary vessels, giving periarterial oedema and intersitial and alveolar infiltrates. Eventually, irreversible interstitial fibrosis arises. |
Sequelae to heartworm infection include pulmonary thromboembolism, which can either occur due to the death and metastasis of adult worms, or due to platelet aggregation induced by the parasite. In severe cases, live nematodes can migrate to the right ventricle, right atrium and caudal vena cava. The resulting incompetence of the tricuspid valve, augmented by concurrent pulmonary hypertension, leads to signs of right-sided heart failure. Flow of erythrocytes through the mass of parasites formed can also cause haemolysis and thus haemoglobinaemia. This combination of acute right-sided heart failure and intravascular haemolysis is referred to as "caval syndrome", which in severe cases can also be characterised by thromboembolic events and [[Disseminated Intravascular Coagulation|disseminated intravascular coagulation]]. Due to the smaller numbers of adult worms, caval syndrome is less common in cats<sup>2</sup>. | Sequelae to heartworm infection include pulmonary thromboembolism, which can either occur due to the death and metastasis of adult worms, or due to platelet aggregation induced by the parasite. In severe cases, live nematodes can migrate to the right ventricle, right atrium and caudal vena cava. The resulting incompetence of the tricuspid valve, augmented by concurrent pulmonary hypertension, leads to signs of right-sided heart failure. Flow of erythrocytes through the mass of parasites formed can also cause haemolysis and thus haemoglobinaemia. This combination of acute right-sided heart failure and intravascular haemolysis is referred to as "caval syndrome", which in severe cases can also be characterised by thromboembolic events and [[Disseminated Intravascular Coagulation|disseminated intravascular coagulation]]. Due to the smaller numbers of adult worms, caval syndrome is less common in cats<sup>2</sup>. | ||
| Line 126: | Line 126: | ||
[http://www.cabi.org/cabdirect/FullTextPDF/2005/20053201370.pdf ''' The utility of echocardiography in the diagnosis of feline heartworm disease: a review of published reports.''' Defrancesco, T. C.; Atkins, C. E.; Seward, R. L.; Knight, D. H.; American Heartworm Society, Batavia, USA, Recent advances in heartworm disease: Symposium '98, Tampa, Florida, USA, 1-3 May, 1998, 1998, pp 103-106, 20 ref.] | [http://www.cabi.org/cabdirect/FullTextPDF/2005/20053201370.pdf ''' The utility of echocardiography in the diagnosis of feline heartworm disease: a review of published reports.''' Defrancesco, T. C.; Atkins, C. E.; Seward, R. L.; Knight, D. H.; American Heartworm Society, Batavia, USA, Recent advances in heartworm disease: Symposium '98, Tampa, Florida, USA, 1-3 May, 1998, 1998, pp 103-106, 20 ref.] | ||
| − | |||
| − | |||
}} | }} | ||
| Line 158: | Line 156: | ||
{{review}} | {{review}} | ||
| − | + | {{OpenPages}} | |
| − | |||
[[Category:Filarioidea]] | [[Category:Filarioidea]] | ||
Revision as of 15:06, 15 October 2013
Also known as: Heartworm Disease — Dirofilariasis
Beware confusing with: Angiostrongylus vasorum, angiostrongylosis.
Introduction
Dirofilaria immitis is a nematode parasite that causes heartworm disease in dogs, cats and ferrets. Heartworm disease is transmitted by mosquito bites and there are more than 70 species of mosquito that are able to transmit infection; Aedes, Anopheles and Culex are the most common vector species. Heartworm disease has been reported in many countries with temperate climate and is particularly prevalent in the USA, Canada, and southern Europe. The introduction of the PETS travel scheme has increased the concern over Dirofilariasis in the UK.
Dirofilaria does have zoonotic potential: infected mosquitos can transmit D. immitis to humans, but the infection does not become patent. The infective larvae instead reach the lungs, become encapsulated, and die causing granulomatous reactions called "coin lesions" in the process. These are only important because they may be confused with neoplastic metastasis to the lungs on radiography1.
Life Cycle
Dirofilaria immitis adults reach maturity and sexually reproduce in the pulmonary arteries and right ventricle. Adult males are around 15cm in length, and females are around 25cm1. After mating, female worms release larvae known as microfilariae (or L1) into the circulation. When a mosquito takes a blood meal from the infected dog or cat, microfilariae are ingested. Mosquitoes are true intermediate hosts for Dirofilaria immitis, since microfilariae require a period of maturation to L2 then L3 in the vector. The duration of this development depends upon environmental conditions. For example, maturation at 30°C takes around 8 days, but when temperatures are down to 18°C, this takes around one month2. Below 14°C, development is halted and resumes when temperatures rise. In cooler climates, this means that transmission of heartworm disease to new canine or feline hosts can only occur in warmer months.
Once matured, L3 in the mosquito migrate to the labium, from which they erupt onto the host's skin as the mosquito feeds. Larvae then migrate into the bite wound and, as most dogs are highly susceptible to heartworm disease, most L3 then establish infection. It takes 2-3 days for L3 to moult to L4, which remain in the subcutaneous tissues for up to two months before becoming young adults (L5) and migrating to the pulmonary arteries.
Cats differ from dogs in that they are more resistant to infection with Dirofilaria immitis. A lower percentage of exposed cats develop adult infections, and when this does occur the burden is usually low1. L5 in the pulmonary arteries also have a relatively short (2 year) survival time in cats.
Pathogenesis
Heartworm disease primarily affects the cardiopulmonary system and the severity and extent of lesions depends on several factors. These include the number and location of adult worms1, 2, the duration of infection, and the level of activity of the host1. Parasites in the pulmonary arteries cause mechanical irritation, leading to endothelial damage, proliferation of the intima and perivascular cuffing with inflammatory cells. This results in narrowing and occlusion of the vessels which in turn causes pulmonary hypertension. A combination of pulmonary hypertension and inflammatory mediators can lead to in an increase in the permeability of pulmonary vessels, giving periarterial oedema and intersitial and alveolar infiltrates. Eventually, irreversible interstitial fibrosis arises.
Sequelae to heartworm infection include pulmonary thromboembolism, which can either occur due to the death and metastasis of adult worms, or due to platelet aggregation induced by the parasite. In severe cases, live nematodes can migrate to the right ventricle, right atrium and caudal vena cava. The resulting incompetence of the tricuspid valve, augmented by concurrent pulmonary hypertension, leads to signs of right-sided heart failure. Flow of erythrocytes through the mass of parasites formed can also cause haemolysis and thus haemoglobinaemia. This combination of acute right-sided heart failure and intravascular haemolysis is referred to as "caval syndrome", which in severe cases can also be characterised by thromboembolic events and disseminated intravascular coagulation. Due to the smaller numbers of adult worms, caval syndrome is less common in cats2.
In cats, heartworm disease generally causes a diffuse pulmonary infiltrate and an eosinophilic pneumonia2. Adult worms may die and embolise to the lungs, resulting in severe haemorrhage and oedema of the affected lobe. Immature nematodes have also been known to migrate to sites other than the pulmonary arteries and heart such as the CNS, eye and subcutaneous tissues. These ectopic infections are far more common in cats than in dogs, suggesting that D. immitis is not well adapted to feline hosts.
Signalment
Dirofilaria immitis infection affects dogs more commonly than cats, and risk is greatest in outdoor animals. Dogs of any age may be affected, but infections are most common in 3 to 8 year old dogs, and medium and large breeds are over-represented1, 3. In cats, there are no breed or age predispositions, but males are more frequently affected3. Ferrets may also contract dirofilariasis; there are no age or sex predilections1.
Diagnosis
Clinical Signs
In dogs, historical findings at the time of presentation can vary. Some animals are asymptomatic, or cough only occasionally. In countries where heartworm is endemic, animals may be routinely tested for dirofilariasis six months after the end of the high-risk season3. Therefore, positive laboratory testing may be the first indication of disease1. More obvious signs may be seen depending on the severity of disease. Generally, the onset of heartworm disease is insidious, and clinical signs are related either to a high parasite burden, or to an allergic response to the parasite2. Affected dogs most often show coughing, and dyspnoea/tachypnoea, exercise intolerance, loss of condition and syncope may also be seen. In severe cases the pulmonary vessels may rupture, leading to haemoptysis or epistaxis. There is a tendency for signs to only manifest during exercise, and so patients with a sedentary lifestyle may never show overt disease. Right-sided congestive heart failure may ensue when worm burden is high, and signs can include jugular distension, ascites, marked exercise intolerance and hepatomegaly. A systolic murmur is sometimes audible on cardiac auscultation.
A classification system for the presentation of heartworm disease exists1, outlined in the table below.
Asymptomatic or mild disease
| |
Moderate disease
| |
Severe disease
| |
Caval syndrome
|
Caval syndrome is a very severe form of heartworm disease that can occur in dogs and cats. It is characterised by respiratory distress, signs of right-sided heart failure, intravascular haemolysis and haemoglobinuria. Disseminated intravascular coagulation frequently occurs, and the syndrome is often fatal.
In cats, most infections are asymptomatic. However, sudden death can occasionally occur. This may be preceded by an acute respiratory crisis, thought to be due to parasitic thromboembolism and obstruction of a major pulmonary artery1, 2. When clinical signs are less acute, they are vague and may include anorexia, weight loss and lethargy. Intermittent coughing and dyspnoea can appear similar to feline asthma. Syncope may also occur, and cats may vomit. The cause of this vomiting is undetermined3.
Radiography
In dogs, thoracic radiography provides good information on disease severity and is useful for screening dogs showing clinical signs compatible with D. immitis infection1. However, thoracic radiograph do not necessarily reflect the current worm burden: radiographic signs of advanced disease can persist long after an infection has run its course4. Conversely, dogs with high burdens may be inactive and thus show few clinical signs or radiographic changes. Radiographic signs are mild-to-moderate in class II disease, but become more obvious in class III infections. The main pulmonary artery is enlarged1, 4, and the caudal lobar vessels appear tortuous1. Ill-defined, fluffy infiltrates are apparent, and often surround the caudal lobar vessels. Right-sided cardiomegaly may be appreciated, and pleural and peritoneal effusions can be noted in right-sided congestive heart failure4.
Cardiac changes on thoracic radiography are less common in cats than dogs. The caudal lobar veins are enlarged (greater than 1.5 times the width of the ninth rib), and the pulmonary arteries are blunted and tortuous3, 5. Patchy parenchymal infiltrates may be seen in the region of vessels in animals showing respiratory signs1, 3. Enlargement of the main pulmonary artery cannot normally be seen in cats, as it has a relatively midline position and is thus obscured by the cardiac silhouette1, 5. Right-sided cardiomegaly is not considered a typical finding in the cat5.
Echocardiography
In dogs, echocardiography is not particularly useful as a diagnostic tool for heartworm disease. In severe, chronic pulmonary hypertension, right ventricular hypertrophy, septal flattening, underloading of the left heart, and high-velocity tricuspid and pulmonic regurgitation may be seen1. With caval syndrome or high-burden infections, worms may be visualised in the right heart and vena cava.
Echocardiography is more important in cats than dogs because of the increased difficulty of diagnosis and the fact that this test can have a high sensitivity depending on operator experience1. Specificity is 100%5, and the test can help exclude or confirm other primary cardiac diseases such as hypertrophic cardiomyopathy3. Worms can be visualised as parallel hyperechoic lines1, and are seen in the right atrium and ventricle and main pulmonary artery1, 3, 5.
Electrocardiography
The ECG of infected dogs is usually normal. Right ventricular hypertrophy patterns may be seen in chronic ,severe pulmonary hypertension and are associated with impending or apparent right-sided congestive heart failure4. Arrhythmias do not normally occur, buy atrial fibrillation is is occasionally seen in Class III disease.
Electrocardiography is less useful in the cat, as involvement of the heart chambers does not occur as frequently as in the dog5.
Laboratory Tests
In both dogs and cats, routine haematology, biochemistry and urinalysis should be performed. Most parameters are usually within normal limits, but an anaemia can often be seen. Eosinophilia and basophilia are also common1, 3. Eosinophilia peaks as L5 enter the pulmonary arteries and subsequently varies. An inflammatory leukogram is possible3. Hyperglobulinaemia due to antigenic stimulation is an inconsistent finding1, 3. Right-sided heart failure or immune-complex glomerulonephritis can lead to hypoalbuminaemia and, very occasionally, nephrotic syndrome1. Because of this, it is possible for urinalysis to reveal proteiunuria1, 3. Haemoglobinaemia and haemoglobinuria are associated with caval syndrome3.
There are several methods for the specific demonstration of Dirofilaria immitis in the animal. Firstly, direct microscopic examination allows rapid identification of microfilariae in a drop of fresh blood, as their movements can vigorously displace the surrounding red blood cells2. Despite being quick, simple and inexpensive, this test is not sufficiently sensitive to provide a definitive diagnosis, particularly when there is a low concentration of microfilariae in the bloodstream. Filtration methods therefore exist to facilitate the microscopic demonstration of microfilariae2, 3. These include the modified Knott's test, which involves haemolysis, centrifugation and staining with methylene blue before direct examination. Tests such as this are more sensitive than merely examining a drop of blood, and the morphology of microfilariae can be clearly seen. However, sensitivity in comparison to other methods is still low and so microfilarial identification tests are often reserved for confirmation of weak positive antigen tests and determination of microfilarial status prior to treatment with a microfilaricide3. Cats frequently lack circulating microfilariae, and so direct microscopic examination is of little use in this species.
Tests exist to detect D. immitis antigens. ELISAs specific for proteins released from the reproductive tract of adult female worms are available for in-house use2. Sensitivity and specificity are excellent, but small worm burdens and the presence of immature female- or male-only infections can give low antigen titres hence false negatives. This is especially common in cats. Specific agglutination and immunochromatography techniques are also available for use in dogs. Any antigen test performed in the first six months of infection may give false negative results as levels of circulating antigen are initially low while female worms mature. In-house tests are also available to detect antibody against Dirofilaria immitis. The presence of antibodies confirms exposure, but does not necessarily provide information about current infection. These tests are therefore most useful for ruling out infection. D. immitis antibody tests have a low specificity2 and so have largely been superceded by tests for antigen.
PCR-based tests are highly sensitive and specific for the diagnosis of immature and adult heartworms, and are especially useful in unconventional (e.g. wildlife) hosts2. At present, these tests are not widely available for the diagnosis of Dirofilaria immitis.
Pathology
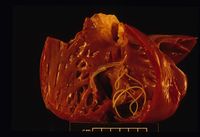
On post-mortem examination, Dirofilaria immitis worms are apparent in the pulmonary artery and possibly the right side of the heart. The right side of the heart is found to be enlarged and there is proliferation of the pulmonary arterial myointima. Pulmonary thromboembolism and haemorrhage may be seen. If right-sided congestive heart failure was present in life, hepatomegaly and hepatic congestion will be apparent.
Treatment
Animals with right-sided congestive heart failure require stablisation with diuretics, ACE inhibitors and cage rest before treatment for heartworm disease is implemented. Animals with severe respiratory signs also require stabilisation with oxygen supplementation, anti-inflammatory doses of corticosteroid and anti-thrombotic drugs.
The specific adulticidal treatment for Dirofilaria immitis is melarsomine dihydrochoride, a new generation arsenical compound. Melarsomine is administered intramuscularly into the epaxial muscles, and pressure should be applied during and after needle withdrawal3. A "graded-kill" protocol is recommended: an initial injection is followed one month later with two injections at an interval of 24 hours, given on opposite sides1-4. This spreads the killing effects over two treatments, with an aim to reducing the occurrence of thromboembolism after parasite death. Cage rest and anti-inflammatory doses of corticosteroids in the week following melarsomine treatment can also reduce the likelihood of pulmonary thromboembolism. Antigen testing four months after adulticidal treatment will determine whether it is necessary to repeat the therapy3.
Adulticidal treatment may be declined by the owner, owing to the risk of thromboembolism. Alternatively, it may not be possible to implement adulticidal treatment if the patient is suffering renal or hepatic failure3. In these cases, monthly administration of prophylactic doses of ivermectin is a reasonable treatment option, as it prevents further infection and may kill some adult nematodes2.
Even low grade infections in cats may result in pulmonary thromboembolism with adulticidal treatment. Because of this, symptomatic treatment of sick cats may be followed by surgical or catheter-based extraction of nematodes once the patient is stable3. Stablisation is similar to that for feline asthma, and can include cage rest, oxygen supplementation, bronchodilators (e.g. theophylline), tapering doses of prednisolone, and balanced fluid therapy if indicated3. Heartworms have a much shorter life-span in cats, and spontaneous remission is seen in some cases. Regular monitoring may therefore be the best course of action in clinically well cats.
In caval syndrome, surgery is the treatment of choice. Worms are removed from the right side of the heart and the main pulmonary artery using flexible crocodile or basket-type retrieval forceps2. This procedure is complex and requires general anaesthesia and fluoroscopic imaging, but reduces the risk of thromboembolism following subsequent adulticidal treatment. Symptomatic and supportive therapy to stabilise the patient should be continued for around one month after surgery before adulticidal treatment is administered3.
No drugs are specifically approved for microfilaricidal treatment of Dirofilaria immitis, and successful elimination of adult worms should result in the demise of circulating microfilariae four to six weeks later2. Single doses of ivermectin, milbemycin oxime, moxidection or selamectin are, however, effective at removing microfilariae from the circulation. The sudden death of large numbers of microfilariae may invoke an anaphylactic response, and oral prednisolone may be administered with microfilaricides to help prevent this.
Heartworm prophylaxis should be implemented in all cats and dogs living in or visiting areas in which Dirofilaria immitis is endemic. Ivermectin or milbemycin oxime can be given per os on a monthly basis, and selemectin spot-on is effective when applied each month. If animals have already been exposed to Dirofilaria immitis it may be wise to perform an antigen test before starting treatment. In endemic countries, routine antigen testing six months after the end of the previous heartworm season will detect infections that have slipped through the net, and enable treatment during the mild, early stages of disease3.
Prognosis
In mildly symptomatic or asymptomatic animals, the course of dirofilariasis is usually uneventful following treatment and the prognosis is excellent3. Animals with severe infection carry a guarded prognosis with a higher risk of complications.
| Dirofilaria immitis Learning Resources | |
|---|---|
 Search for recent publications via CAB Abstract (CABI log in required) |
Dirofilaria immitis publications since 2000 |
 Full text articles available from CAB Abstract (CABI log in required) |
A review of American heartworm society guidelines for the management of heartworm infections in cats. Guerrero, J.; The North American Veterinary Conference, Gainesville, USA, Small animal and exotics. Proceedings of the North American Veterinary Conference, Orlando, Florida, USA, 16-20 January 2010, 2010, pp 1173-1176, 1 ref. |
| Sample Book Chapters | ||||
|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
Links
- The Merck Veterinary Manual - Heartworm Disease
- dogheartworm.org
- DEFRA - Dog and Cat Travel and Risk Information
References
- Merck & Co (2008) The Merck Veterinary Manual (Eighth Edition), Merial.
- Ferasin, L (2004) Disease risks for the travelling pet: Heartworm disease, In Practice, 26(6), 350-357.
- Tilley, L P and Smith, F W K (2004) The 5-minute Veterinary Consult (Fourth Edition),Blackwell.
- Venco, L (2007) Heartworm (Dirofilaria immitis) disease in dogs. Dirofilaria immitis and D. repens in dog and cat and human infections, 117-125.
- Venco, L (2007) Heartworm (Dirofilaria immitis) disease in cats. Dirofilaria immitis and D. repens in dog and cat and human infections, 126-132.
- Ridyard, A (2005) Heartworm and lungworm in dogs and cats in the UK, In Practice, 27(3), 147-153.
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
Error in widget FBRecommend: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a0d8ea61a017_71095315 Error in widget google+: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a0d8ea7ecdf3_90667438 Error in widget TwitterTweet: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a0d8ea95e680_51324276
|
| WikiVet® Introduction - Help WikiVet - Report a Problem |