| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
Also known as antibodies
Introduction
Also called antibodies
Immunoglobulins (Ig) are the soluble form of B cell receptors (BCR) released by plasma cells after they have been activated. Immunoglobulins have to bind to a number of different antigens in a variety of environments and as such there are several different immunoglobulin classes. Each class has an optimum environment of action.
Structure
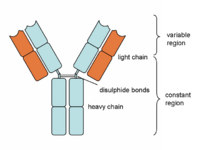
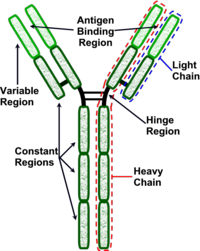
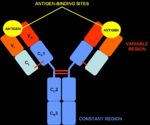
Antibodies are "Y-shaped" and consist of two identical heavy (H) chains and two identical light (L) chains with each H chain being linked to an L chain. The heavy chain consists of 4 or 5 protein domains, 1 amino terminal variable domain and 3 or 4 constant domains. Each light chain has one variable and one constant domain. Each arm of the molecule consists of one L chain and part of an H chain and there are two identical antigen-binding sites on each antibody molecule, one at the tip of each arm. The binding sites are made up of a combination of the variable domains of 1H and 1L, although single cells only have receptors of one specificity. The antigen binding site of an antibody is determined by a combination of the amino acid sequence of both the heavy and light chain variable domains (VH and VL). Antibodies of different specificities have different amino acids within one of three regions of the variable domains and these are called the hypervariable regions. The variable domains are folded in such a way as to form a pocket into which antigens fit. The constant domains do not bind antigen, although they determine the biological functions of the antibody molecules.
At the carboxyl terminus of B-cell expressed Ig is a region that holds the antibody molecule in the cell membrane. Secreted 'free' antibodies do not possess this region. The Fab fragments of the immunoglobulin is the antigen binding fragment generated from the light chains when digested by papain (proteolytic enzyme). The Fc fragment is the fragment produced by papain from the heavy chains and this fragment mediates phagocytosis, triggers inflammation and targets Ig to certain tissues.
Function
- For an overview of the role of immunoglobulins in the adaptive immune response, see here
- All antibody classes bind antigen in a similar manner:
- The initial trigger for all antibody function is the association of antigen with the antigen-binding sites of the immunoglobulin molecules
- However, different classes serve different physiological functions
- Functional differences between antibody classes are reflected in the structural differences in their heavy chain constant regions
- These regions comprise the effector domains of antibody molecules
- Antibodies have four major effector functions:
Blockade and agglutination
- The action of antibody binding to antigen can be protective
- Antibody may bind to biologically active sites on toxins, inhibiting their binding sites
- In a similar manner, antibody can bind the surface of viruses and prevent them from infecting cells
- Antibody molecules are at least divalent, and many are greater than divalent
- This means that antibody can cross-link antibody: agglutination
- The ability to cross-link can be the primary function of the antibody
- This means that antibody can cross-link antibody: agglutination
- In order to be pathogenic, many micro-organisms must be able to contact viable host cells
- If antibody molecules agglutinate these micro-organisms, cell contact is prevented
- The pathogenic effect is removed or reduced
- If antibody molecules agglutinate these micro-organisms, cell contact is prevented
- The main biological function of IgA is to cross-link potentially pathogenic molecules
Promotion of phagocytosis
- Phagocytes hace receptors for the Fc portion of antibody
- Fc receptors - FcR
- FcR bind the Fc portion of antibody on antigen/antibody complexes
- Antibody alone does not interact with FcR
- Interaction of FcR with Ab/Ag complexes stimulates:
- Cellular activation
- Causes greatly increased efficiency of phagocytosis - opsonisation
- Production of intracellular enzymes
- Promotes the killing and digestion of internalised micro organisms
- Systems important in intracellular killing include:
- Free radicals
- Oxygen and chlorine free radicals
- Hydrogen peroxidase
- Nitric oxide
- Produced by nitric oxide synthase
- Free radicals
- Secretion of certain enzymes and cytotoxic molecules
- The degranulation of phagocytes
- Cellular activation
- These systems are also activated by the interferons, especially the immune interferon – IFNγ
- Antibody-mediated opsonisation of micro organisms is much more efficient that innate receptors
- Phagocytes also have receptors for iC3b, a complement component
- A major initiator of opsonisation
Degranulation of mast cells/ eosinophils
Complement fixation
Classes
There are five classes of Immunoglobulins, which vary due to the composition of their heavy chains. These are common across mammals but subclasses of them do vary between species. IgY is present in yolk.
| IgA | IgD | IgE | IgG | IgM | |
|---|---|---|---|---|---|
| Weight (kDa) | 360 | 180 | 200 | 180 | 900 |
| No. subunits | 2 | 1 | 1 | 1 | 5 |
| Heavy chain | α | δ | ε | γ | µ |
| Mainsite of production | Alimentary/Respiratory tracts | Spleen/Lymph nodes | Alimentary/Respiratory tracts | Spleen/Lymph nodes | Spleen/Lymph nodes |
Variation
Subclasses
Subclasses of the five major classes also exist. These occur in the heavy chains and are coded for by the IGH gene. For examples horses have six subclasses of the IgG immunoglobulin coded for by the genes IGHG1→ IGHG6 while cattle and sheep only have three subclasses (IGHG1→ IGHG3).
- Within the IgG class there are several subclasses depending on the species
- Humans and rodents have four IgG subclasses
- Dogs have three subclasses
- Ruminants have two or three subclasses
- In some species there are also two IgA subclasses
- Specificity is unrelated to class
- Different classes of antibody can be associated with the same V domains and have the same specificity
- It is possible for a single B-cell to produce antibody of one specificity but two or more classes
- A mature B-cell has the genetic capacity to produce antibody of all classes and subclasses but of one specificity only
Allo & Idiotypes
Allotypes are differences in immunoglobulins between individuals of a particular species. They are inheritable. Idiotypes are variations in the sequences of amino acids in the variable regions of the light and heavy chains.
Immunoglobulin Class Switching
- Different classes of antibody differ from each other in:
- Size
- Charge
- Amino acid composition
- Associated carbohydrate
- Function
- Immunoglobulin heavy and light chains are encoded by separate genes
- The first 300 bases of each gene encodes the variable part of each protein chain
- The combination of the VH and VL contribute to the antigenic specificity
- Any individual has the capability of producing over 100 million different antibody specificities
- The combination of the VH and VL contribute to the antigenic specificity
- The rest of the antibody gene encodes all the constant domains.
- The first 300 bases of each gene encodes the variable part of each protein chain
- The sequence of constant region genes on the chromosome is M, G, E and A
- In humans/ rodents the Cδ (IgD) is immediately downstream of the M gene
- Prior to antigenic stimulation, B-cells express cell membrane-associated IgM
- The first immunoglobulin produced during an immune response is always IgM because the Cμ gene is the first constant H chain gene downstream of the the variable domain
- After antigenic exposure the IgM+ B-cells differentiate
- Begin to synthesise other classes of immunoglobulin
- Only under the direct influence of a T-cell
- Known as immunoglobulin heavy chain switch
- Retain the same variable domains expressed by the parental B-cell
- Begin to synthesise other classes of immunoglobulin
- There are also two possible light chains
- There are no functional consequences for either type
- The IgG antibodies produced in both primary and secondary responses originate from the same clones of B-cells as the IgM antibodies
- The different antibody classes have the same variable domain combinations, and therefore the same antigenic specificity
- IgE and IgA producers can be generated
- These immunoglobulins tend to be produced within lymphoid tissue associated with mucosal surfaces
Clinical Uses
Immunoglobulins Flashcards
Links
Websites