Difference between revisions of "Adrenal Glands - Pathology"
m |
m |
||
| Line 8: | Line 8: | ||
<br> | <br> | ||
==Anatomy and Physiology== | ==Anatomy and Physiology== | ||
| − | Anatomy and Physiology of the adrenal glands can be found [[ | + | Anatomy and Physiology of the adrenal glands can be found [[Pituitary Gland - Anatomy & Physiology|here]]. |
==Functional anatomy== | ==Functional anatomy== | ||
[[Image:Normal adrenal cortex.jpg|right|thumb|125px|<small><center>'''Normal Adrenal Cortex'''. Courtesy of A. Jefferies</center></small>]] | [[Image:Normal adrenal cortex.jpg|right|thumb|125px|<small><center>'''Normal Adrenal Cortex'''. Courtesy of A. Jefferies</center></small>]] | ||
Revision as of 16:33, 3 September 2008
|
|
Anatomy and Physiology
Anatomy and Physiology of the adrenal glands can be found here.
Functional anatomy
The adrenal glands are essential to life. They are closely applied to the cranial poles of each kidney. Consists of a cortex and medulla, each having different embryological origin. In some lower animals the two components actually exist as separate endcrine glands.
- Cortex: Secretes a variety of steroid hormones derived from cholesterol.
- Zona glomerulosa: Secretes Aldosterone. Concerned with electrolyte and fluid homeostasis.
- Zona Fasciculata: Secretes Glucocorticoids. Controls metabolism of lipid, protein and carbohydrate.
- Zona Reticularis: Produces Sex hormones. Supplements gonadal sex hormone secretion.
- Medulla: Embryologically similar origin to the sympathetic nervous system. Secretes catecholamine hormones E.g. Adrenaline, noradrenaline.
Secretion from the medulla is directly controlled by the sympathetic nervous system, allowing a rapid response. In contrast, the secretions from the cortex are controlled by the hormones ACTH and other circulating hormones.
As with any endocrine gland there are two main types of pathology:
- Insufficiency leading to Addisons disease.
- Excess leading to Cushings disease.
Adrenal Hypofunction
Primary Hypoadrenocorticism
Multiple aetiologies, including:
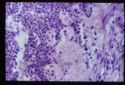
- Adrenal atrophy: Adrenals are grossly small and difficult to find. They are dark brown on cut section. On histology there will be an infiltrate of lymphocytes, plasma cells and macrophages. Thought to be auto-immune, also see increased incidence of other immune mediated diseases E.g. IMHA.
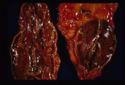
- Adrenal necrosis: May be:
- Infectious in aetiology E.g Salmonellosis in horses. Necrosis may also occur due to:
- Myoarteritis in uraemic animals resulting in ischaemia of the adrenal gland.
- Another possible type of necrosis is idiopathic.
All are seen grossly as areas of red haemorrhage and yellow necrotic foci.
- Bilateral adrenalectomy/Mititane therapy: Mitotane selectively destroys the zonas fasciculata and reticularis while sparing the essential zona glomerulosa. Used in the treatment of Cushings disease. Iatrogenic Addisons disease is a common sequale of treatment.
Secondary Hypoadrenocortisism
Deficient pituitary secretion of ACTH. Often iatrogenic due to withdrawal of glucocorticoid treatment. Prolonged high dose treatment induce adrenal atrophy due to the effect of negative feedback on the pituitary. The withdrawal of drug must be gradual to allow to adrenal gland to return to function over a period of time. Usually little effect on mineralocorticoids as ACTH has little trophic effect on their production.
Pathophysiology
- Aldosterone deficiency: Aldosterone normally acts to aid sodium reabsorption and potassium excretion in the kidney. With deficient aldosterone the animal will have:
- Hyponatraemia.
- Hypochloraemia.
- Hyperkalaemia.
- Glucocorticoid deficiency: Cortisol is the stress hormone which acts to increase blood glucose in stressful situations (amongst other functions). Deficiency leads to the following:
- Hypoglycaemia.
- Increased circulating lymphocytes and eosinphils due to removal of the immunosuppressive glucocorticoids.
- Increased skin pigmentation; Low levels of glucocorticoids allow increased ACTH production as negative feedback on the pituitary is removed/decreased. As ACTH is released, so is MSH therefore a change in skin pigmentation indicates endocrine disease.
Clinical signs:
- Acute necrosis will present as an acute syndrome with hypovolaemic shock, vomiting and collapse.
- Chronic damage to the adrenal gland will result in dehydration, diarrhoea, anorexia and weakness.
Diagnosis:
- Haematology:
- Haemoconcentration in acute crisis; due to rapid dehydration.
- Non-regenerative anaemia in chronic form; glucocorticoid deficiency decreases erythropoeisis.
- Electrolyte imbalance: as above.
- Pre-renal azotaemia: elevated urea and creatinine.
- ACTH stimulation test: Positive test is low initial cortisol with no response to ACTH.
- ECG change: Due to hyperkalaemia. In severe cases may see P-wave absence and sino-atrial standstill.
Treatment:
- Acute crisis: Rapid i/v saline and i/v glucocorticoids.
- Chronic form: Fludrocortisone acteta to replace mineralocorticoids. Add table salt to food and give glucocorticoids in times of stress E.g. transport.
Adrenal Hyperfunction
Cushings disease: Most often seen in the dog.
Aetiology:
Pituitary Dependant
80-85% cases show bilateral adrenal hyperplasia due to excess stimuation by ACTH. There is failure of negative feedback at the level of the pituitary and so ACTH is produced in an unregulated fashion. Thought to be due to a functional chromophobe cell (Produces ACTH and MSH) neoplasia, although visible macroadenomas are only found in 10-15% cases with this aetiology. Most cases are therefore thought to be microadenomas and may be visualised by histopathological staining of the pituitary.
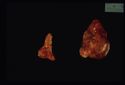
Grossly the adrenals have an irregular surface with protruding nodules of cortical tissue; the hyperplased zona fasciculata cells.
Adrenal Dependant
Approximately 15% all cases of Cushings disease. Of the adrenal neoplasia approximately 50% are benign and 50% are malignant.
Iatrogenic
Due to excessive administration of parenteral corticosteroids. In these cases there will be adrenal atrophy as the administered steroids have a negative feedback effect on the pituitary and ACTH release is inhibited.
Pathophysiology
The clinical signs of Cushings disease are brought about by excess glucocorticoids, particularly cortisol.
- Liver: Cortisol induces enzymes to increase gluconeogenesis leading to hyperglycaemia in Cushings patients. The pancreas attempts to maintain normal blood glucose by producing increasing amount of insulin. Eventually the pancreas is exhausted inducing a diabetic state.
- Muscle: Cortisol stimulates protein catabolism for gluconeogenesis so patients exhibit muscle weakness.
- Skin: Again protein catabolism and weakness giving poor wound healing.
- Calcinosis cutis: Collagen damage due to protein catabolism allows the deposition of calcium in the skin. Calcium acts as a foreign body producing a granulomatous reaction.
- MSH production stimulates the pigmentation of the skin with melanin.
- Cortisol inhibits the growth phase of the hair cycle (ANAGEN) so hair growth stops. When epilated over areas of high friction there will be bilaterally symmetric non-pruritic alopecia.
- Fat: Lipolysis is stimulated by cortisol to provide precursors for gluconeogenesis. Seen clinically as a redistribution of fat to the abdomen and back of the neck; Pot-bellied appearance.
- Immune system: Cortisol is anti-inflammatory by a number of mechanisms E.g. stabilising lysosomal membranes. The circulation will contain fewer lymphocytes and eosinophils.
- Kidney: Cortisol interferes with ADH action on the kidney resulting in polyuria and polydipsia.
Diagnosis
- ACTH Stimulation test: Measure Cortisol before and 30-60 mins after i/v Synacthen. Positive result is initially high cortisol followed by a markedly elevated cortisol after stimulation (>600nmol/l).
Adrenal dependant disease may be unresponsive to ACTH.
- Low dose dexamethasone test: Sample before and 3 and 8 hours after i/v dexamethasone at 0.01mg/kg. Normal and dogs with pituitary dependant disase show suppression of cortisol production to <50% at 3 hours whereas dogs with adrenal dependant disease have high cortisol levels which are not suppressed.
- High dose dexamethasone test: Distinguishes pituitary and adrenal dependant disease once Cushings has been diagnosed. Dexamethasone dose is 0.1mg/kg. Pituitary dependant disease will show suppression of cortisol production due to negative feedback at the pituitary whereas adrenal dependant disease will not.
Treatment
- Mitotane: Selectively destroys the zona fasciculata and zona reticularis while sparing the zona glomerulosa. Need to use an induction dose followed by a maintenance dose.
- Trilostane: Synthetic steroid analogue. Competitively inhibits enzymes of sterois synthesis.
- L-Deprenyl: Monoamine oxidase inhibitor. Increases dopamine input to hypothalamus and pituitary and so inhibits ACTH secretion.
Pituitary tumours may be treated with radiotherapy or surgery.