Difference between revisions of "Immunity to Parasites"
Rjfrancisrvc (talk | contribs) |
Rjfrancisrvc (talk | contribs) |
||
| (4 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Innate Immunity== | ==Innate Immunity== | ||
| − | [[File: | + | [[File:Helminth Killing edit.png|300px|right|thumb|Helminth Killing - R.J.Francis, RVC 2012]] |
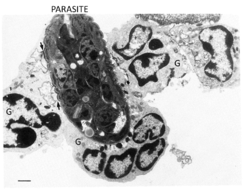
| − | [[File:Helmith Killing by Granulocytes.png|250px|right|thumb| | + | [[File:Helmith Killing by Granulocytes.png|250px|right|thumb|Electron Micrograph of Helminth Killing by Granulocytes (G). Adapted from [[http://eprints.adm.unipi.it/527/]] - R.J.Francis, RVC 2012]] |
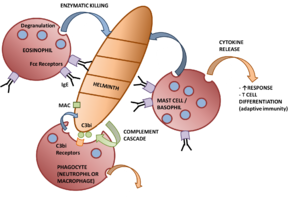
The first line of defence against parasitic infection are the effector mechanisms of the innate immune system. The '''[[Macrophages|macrophages]]''' are important players in the defence against extracellular parasites. This is because macrophages are able to secrete [[Cytokines|cytokines]] as well as perform [[Phagocytosis|phagocytosis]]. In this they can act as 'killer cells' through antibody-dependent cell-mediated cytotoxicity, for example specific [[Immunoglobulins|IgG]]/[[Immunoglobulins|IgE]] enhances the ability of macrophages to kill schistosomules through the interaction of Fc receptors on the surface of the macrophage. Of the secreted cytokines, the secretion of TNFα is of particular importance. This is because TNFα activates other macrophages and can have toxic effects in high amounts. TNFα also renders hepatocytes resistant to malarial infection when in conjunction with IL-1. Cytokine secretion (in particular IFNγ) can also enhance killing by mechanisms using free radicals and O<sub>2</sub>-independent toxins (e.g. nitric oxide). | The first line of defence against parasitic infection are the effector mechanisms of the innate immune system. The '''[[Macrophages|macrophages]]''' are important players in the defence against extracellular parasites. This is because macrophages are able to secrete [[Cytokines|cytokines]] as well as perform [[Phagocytosis|phagocytosis]]. In this they can act as 'killer cells' through antibody-dependent cell-mediated cytotoxicity, for example specific [[Immunoglobulins|IgG]]/[[Immunoglobulins|IgE]] enhances the ability of macrophages to kill schistosomules through the interaction of Fc receptors on the surface of the macrophage. Of the secreted cytokines, the secretion of TNFα is of particular importance. This is because TNFα activates other macrophages and can have toxic effects in high amounts. TNFα also renders hepatocytes resistant to malarial infection when in conjunction with IL-1. Cytokine secretion (in particular IFNγ) can also enhance killing by mechanisms using free radicals and O<sub>2</sub>-independent toxins (e.g. nitric oxide). | ||
<br/> | <br/> | ||
| Line 23: | Line 23: | ||
*Activating [[Complement|complement]], and the subsequent Membrane Attack Complex | *Activating [[Complement|complement]], and the subsequent Membrane Attack Complex | ||
*Blocking attachment to host cells | *Blocking attachment to host cells | ||
| − | *Enhancing macrophage phagocytosis | + | *Enhancing macrophage [[Phagocytosis|phagocytosis]] |
*Involvement in antibody-dependent cell-mediated cytotoxicity | *Involvement in antibody-dependent cell-mediated cytotoxicity | ||
Latest revision as of 12:04, 28 May 2012
Innate Immunity
The first line of defence against parasitic infection are the effector mechanisms of the innate immune system. The macrophages are important players in the defence against extracellular parasites. This is because macrophages are able to secrete cytokines as well as perform phagocytosis. In this they can act as 'killer cells' through antibody-dependent cell-mediated cytotoxicity, for example specific IgG/IgE enhances the ability of macrophages to kill schistosomules through the interaction of Fc receptors on the surface of the macrophage. Of the secreted cytokines, the secretion of TNFα is of particular importance. This is because TNFα activates other macrophages and can have toxic effects in high amounts. TNFα also renders hepatocytes resistant to malarial infection when in conjunction with IL-1. Cytokine secretion (in particular IFNγ) can also enhance killing by mechanisms using free radicals and O2-independent toxins (e.g. nitric oxide).
As well as the macrophages, the granulocytes form the main effector response to parasitic infection. Eosinophils are very important in the destruction of larger parasites even though they are less phagocytic than neutrophils. Most activity from eosinophils is controlled by antigen-specific mechanisms, for example binding to worms coated with IgG/IgE increases degranulation. Degranulation, through the process known as exocytosis, releases enzymes that degrade the parasite into smaller chunks so it can be cleared by phagocytosis. The eosinophils also form the end point of the adaptive immune response to larger parasites with the killing of some larvae being enhanced by the activity of mast cells, for example antigens released by S. mansoni cause IgE-dependent degranulation of mast cells, the products of which selectively attract eosinophils. Mast cells and eosinophils degranulation enhances the TH2 response through the release of cytokines, for example IL-4 and IL-13, that is needed to clear a stubborn infection.
The other granulocytes present in parasite-infected inflammatory lesions and involved in the anti-parasitic response are the neutrophils. In the parasitic response they have similar properties to macrophages with activation caused by cytokines such as TNFα, IFNγ and GM-CSF. Their mechanism for killing is by an intense respiratory burst, with extracellular killing being mediated by hydrogen peroxide (H2O2). In addition, they also express Fc and complement receptors so can participate in antibody-dependent cell-mediated cytotoxicity and phagocytosis. Due to innate inflammatory mechanisms neutrophils are early responders to parasite infection.
Platelets also form part of the innate immune response to parasites. Their main mode of action is the binding and release of granular contents, in particular inflammatory peptides. The cytotoxic activity of plateltes is increased by cytokines such as TNFα and IFNγ. The potential targets include the larval stage of flukes, e.g. T. gondii, T. cruzi and S. Mansoni. Like other effector cells, platelets express Fc receptors, making them able to perform antibody-dependent cytotoxicity.
Adaptive Immunity
Although the innate immune system provides an effective first line of defence, T cells are fundamental in the development of immunity, demonstrated using T-cell deprived mice that fail to resolve otherwise non-lethal infections of, for example, T. cruzi.
- Both CD4+ and CD8+ cells are required for protection, e.g CD4+ cells protect against the blood stage of a Plasmodium infection (erythrocytes do not express MHC class I), while CD8+ cells are required to mediate immunity against the liver stage (hepatocytes do not express MHC class II).
- TH1 cells are required to fight intracellular protozoa - the release of IFNγ activates macrophages to kill the protozoa residing within them
- Helminth infections require both TH1 and TH2 responses, e.g. during S. mansoni the secretion of IFNγ by TH1 cells activates mechanisms that destroy larvae in the lungs, although the TH2 subset, secreting IL-5, predominate. IL-5 is responsible for the eosinophilia associated with parasite infections.
- TH2 cells are required for the destruction of intestinal worms, where they induce mucosal mast cells and interact with eosinophils
While cell-mediated immunity is important in tissue infections, such as Leishmania, specific antibodies are important in controlling parasites that live in the bloodstream, e.g. malaria. Mechanisms of antibody-mediated immunity include:
- Directly damaging protozoa
- Activating complement, and the subsequent Membrane Attack Complex
- Blocking attachment to host cells
- Enhancing macrophage phagocytosis
- Involvement in antibody-dependent cell-mediated cytotoxicity
Immunopathology
The immunopathology is the damage resulting from an immune response to the parasites. Examples include:
- The increase in macrophages and lymphocytes in the liver and spleen can lead to swelling of these organs, e.g. visceral leishmaniasis
- T-cell dependent (TH1 and TH17) granulomas forming in organs, e.g. schistosomiasis in the liver
- The pathology of elephantiasis is thought to be due to changes in the adult filariae in the lymphatic system
- Formation of immune complexes, e.g. deposition in the kidney during malarial infection
- Anaphylactic shock caused by IgE production, e.g. after the rupture of hydatid cysts
- Cross-reaction of antibodies with host tissue, e.g. O. volvulus, the cause of river blindness, expresses an antigen similar to a protein in the retina
- Excessive production of cytokines, such as TNFα, may contribute to pathology of diseases such as malaria
Evading immune defences
Parasites can evade an immune response from the host by changing the antigens presented to the host, produce antigens that mimic the host's antigens and can produce down-regulating factors which suppress or modify the host's immune responses. Having a rapid turnover of their surface coat when host cells bind and by being able to live in sites which are protected from the host's immune response allow parasites to establish themselves in a particular species.
- Selection of innapropriate defences - by exploiting the 'adjuvant' mechanism, some parasites are able to activate the inappropriate helper T cell subset, e.g Leishmania
- Antigenic variation avoids recognition by antibody and complement, e.g. T. brucei
- Inhibiting fusion of lysosomes
- Escaping into the cytoplasm, e.g. T. cruzi
- Inhibiting respiratory burst, e.g. Leishmania
- Forming cysts in muscle tissue, e.g. T. spiralis - also develops decay accelerating factor (DAF)
- Production of antioxidants, e.g. W. bancrofti
Also see Adaptive Immunity to Parasites