Difference between revisions of "Brucella species - Overview"
| (9 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{OpenPagesTop}} |
| + | {{Taxobox | ||
| + | |name =''Brucella'' | ||
| + | |phylum =Proteobacteria | ||
| + | |class =Alpha Proteobacteria | ||
| + | |order =Rhizobiales | ||
| + | |family =Brucellaceae | ||
| + | |genus =Brucella | ||
| + | }} | ||
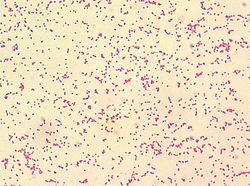
| − | [[File:Brucella.jpg|right|thumb|250px|<small><center> Brucella spp. (Larry Stauffer 2002, Wikimedia commons)</center></small>]] | + | [[File:Brucella.jpg|right|thumb|250px|<small><center> ''Brucella'' spp. (Larry Stauffer 2002, Wikimedia commons)</center></small>]] |
==Overview== | ==Overview== | ||
| − | The '' | + | The ''Brucella'' species cause chronic granulomatous diseases including [[Human Brucellosis|human brucellosis]]. They target reproductive organs of certain species. Infected animals act as reservoirs of infection and organisms can remain viable in moist environment for months. They can also cause undulant fever in humans. |
==Characteristics== | ==Characteristics== | ||
| − | ''Brucella'' species are small, non-moltile, Gram negative coccobacilli. They are facultative intracellular pathogens. ''Brucella'' bacteria are aerobic and capnophilic. They are catalase positive and oxidase and urease positive, except for ''Brucella ovis''. They are modified Ziehl-Neelsen positive, forming clusters of red coccobacilli on smears. Some of the species require enriched media for growth. They are also non-haemolytic. | + | ''Brucella'' species are small, ''non-moltile, Gram negative coccobacilli'''. They are '''facultative intracellular''' pathogens. ''Brucella'' bacteria are '''aerobic'' and '''capnophilic'''. They are '''catalase positive''' and '''oxidase and urease positive''', except for ''Brucella ovis''. They are '''modified Ziehl-Neelsen positive''', forming clusters of red coccobacilli on smears. Some of the species require enriched media for growth. They are also '''non-haemolytic'''. |
| − | Smooth colonies of ''[[Brucella abortus|B. abortus]], [[Brucella melitensis|B. melitensis]]'' and ''[[Brucella suis|B. suis]]'' are small, glistening, blue and translucent after incubation for 3-5 days, and become opaque with age. Rough colonies of ''B. ovis'' and ''[[Brucella canis|B. canis]]'' are dull, yellow, opaque and friable. Slide agglutination with | + | Smooth colonies of ''[[Brucella abortus|B. abortus]], [[Brucella melitensis|B. melitensis]]'' and ''[[Brucella suis|B. suis]]'' are small, glistening, blue and translucent after incubation for 3-5 days, and become opaque with age. Rough colonies of ''[[Brucella ovis|B. ovis]]'' and ''[[Brucella canis|B. canis]]'' are dull, yellow, opaque and friable. Slide agglutination with specific antisera can detect important antigens. Oxidative metaboloic rates can differentiate between species and ''[[Brucella abortus|B. abortus]]'' is lysed by specific bacterophages. |
==Pathogenesis== | ==Pathogenesis== | ||
| Line 19: | Line 27: | ||
==Diagnosis== | ==Diagnosis== | ||
| − | ''Brucella'' species can be detected by serological testing of milk (Milk Ring Test) and beef cattle (Rose Bengal Plate Test). | + | ''Brucella'' species can be detected by '''serological testing of milk (Milk Ring Test) and beef cattle (Rose Bengal Plate Test)'''. |
The serological tests detect anti-lipopolysaccharide antibodies. The LPS antigen is present in virulent as well as some vaccine strains therefore vaccination may confuse serological testing. False positives may be seen due to cross-reaction with LPS in other bacteria. Modified Ziehl-Neelson stains reveal organisms in samples from cotyledons, uterine discharge and foetal abomasal contents. PCR is used for detection in tissue. | The serological tests detect anti-lipopolysaccharide antibodies. The LPS antigen is present in virulent as well as some vaccine strains therefore vaccination may confuse serological testing. False positives may be seen due to cross-reaction with LPS in other bacteria. Modified Ziehl-Neelson stains reveal organisms in samples from cotyledons, uterine discharge and foetal abomasal contents. PCR is used for detection in tissue. | ||
Enriched media is used for isolation along with a complement fixation test. Indirect and competitive ELISA, Serum agglutination test and antiglobulin tests can also be used. | Enriched media is used for isolation along with a complement fixation test. Indirect and competitive ELISA, Serum agglutination test and antiglobulin tests can also be used. | ||
| − | + | <big>'''[[:Category:Brucella_species|See here for a list of ''Brucella'' species]]'''</big> | |
| − | |||
| − | [ | + | {{Learning |
| − | [[Category: | + | |literature search = [http://www.cabi.org/cabdirect/FullTextPDF/2007/20073230792.pdf ''' Epidemiology and epizootology of brucellosis: a review.''' Gul, S. T.; Khan, A.; Faculty of Veterinary Science, University of Agriculture, Faisalabad, Pakistan, Pakistan Veterinary Journal, 2007, 27, 3, pp 145-151, many ref.] |
| + | }} | ||
| + | |||
| + | |||
| + | {{review}} | ||
| + | |||
| + | {{OpenPages}} | ||
| + | |||
| + | [[Category:Brucella_species|A]] | ||
| + | [[Category:Expert_Review]] | ||
Latest revision as of 16:50, 5 July 2012
| Brucella | |
|---|---|
| Phylum | Proteobacteria |
| Class | Alpha Proteobacteria |
| Order | Rhizobiales |
| Family | Brucellaceae |
| Genus | Brucella |
Overview
The Brucella species cause chronic granulomatous diseases including human brucellosis. They target reproductive organs of certain species. Infected animals act as reservoirs of infection and organisms can remain viable in moist environment for months. They can also cause undulant fever in humans.
Characteristics
Brucella species are small, non-moltile, Gram negative coccobacilli. They are facultative intracellular pathogens. Brucella bacteria are aerobic and capnophilic. They are catalase positive and oxidase and urease positive, except for Brucella ovis. They are modified Ziehl-Neelsen positive, forming clusters of red coccobacilli on smears. Some of the species require enriched media for growth. They are also non-haemolytic.
Smooth colonies of B. abortus, B. melitensis and B. suis are small, glistening, blue and translucent after incubation for 3-5 days, and become opaque with age. Rough colonies of B. ovis and B. canis are dull, yellow, opaque and friable. Slide agglutination with specific antisera can detect important antigens. Oxidative metaboloic rates can differentiate between species and B. abortus is lysed by specific bacterophages.
Pathogenesis
The Brucella species penetrate nasal, oral or pharyngeal mucosa where they are phagocytosed and carried to regional lymph nodes. Smooth organisms survive and multiply in cells of the reticulo-endothelial system and inhibit lysosome-phagosome fusion. The bacteria produce superoxide dismutase and catalase that may resist oxidative killing. The lymph nodes enlarge (lymphatic and lymphoreticular hyperplasia) and inflammation is induced. Surviving organisms spread to other organs (liver, spleen, placenta) and cause granulomatous reactions.
Eythritol is a growth stimulant and attracts the bacteria to the placenta of cattle, sheep, goats and pigs. It is also found in mammary glands and the epididymis, which are targets for brucellae. Brucella species can cause infection of foetus and abortion. They can also localise in joints or intervertebral discs in chronic infections. Brucellae that lack outer membrane LPS (rough colonies) are less virulent than those which possess it.
Diagnosis
Brucella species can be detected by serological testing of milk (Milk Ring Test) and beef cattle (Rose Bengal Plate Test). The serological tests detect anti-lipopolysaccharide antibodies. The LPS antigen is present in virulent as well as some vaccine strains therefore vaccination may confuse serological testing. False positives may be seen due to cross-reaction with LPS in other bacteria. Modified Ziehl-Neelson stains reveal organisms in samples from cotyledons, uterine discharge and foetal abomasal contents. PCR is used for detection in tissue. Enriched media is used for isolation along with a complement fixation test. Indirect and competitive ELISA, Serum agglutination test and antiglobulin tests can also be used.
See here for a list of Brucella species
| Brucella species - Overview Learning Resources | |
|---|---|
 Search for recent publications via CAB Abstract (CABI log in required) |
Epidemiology and epizootology of brucellosis: a review. Gul, S. T.; Khan, A.; Faculty of Veterinary Science, University of Agriculture, Faisalabad, Pakistan, Pakistan Veterinary Journal, 2007, 27, 3, pp 145-151, many ref. |
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
Error in widget FBRecommend: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a100e5334453_31838275 Error in widget google+: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a100e54163f9_13118079 Error in widget TwitterTweet: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt69a100e5529526_70010708
|
| WikiVet® Introduction - Help WikiVet - Report a Problem |