T cell differentiation
Introduction
Within the blood and lymphoid organs the majority of CD4+ T cells are antigen-naive T-cells. There is only a small proportion of memory T-cells.
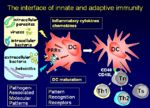
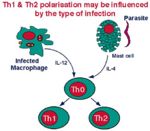
Naive T-cells have yet to encounter antigen and can only be activated by antigen presented by dendritic cells. After initial antigenic activation, naïve T-cells develop into an intermediate stage cell called the TH0 cell which can then be activated by any antigen-presenting cell, e.g. Dendritic cells, macrophages or B-cells.
The TH0 cells have the capacity to differentiate into TH1 and TH2 cells. The type of cell that develops depends on the antigen presenting cell. Macrophages cause the TH0 cell to develop into TH1 cells caused by IL-12 production following macrophage-antigen interaction. B-cells cause the TH0 cell to develop into TH2 cells caused by IL-10 production following B-cell-antigen interaction. On antigenic stimulation the TH1 or TH2 cells become activated, undergo clonal expansion and secrete a range of different cytokines.
For any one cell the cytokine-secreting activation state is short-lived, lasting between 4 - 40 hours. After this time these cells either die, or mature into the long-lived memory cells. The proliferation of T cells continues until antigen disappears.
Dendritic Cells
- There are two different lineages of dendritic cells:
- From myeloid precursor cells
- From plasmacytoid precursor cells
- Stimulate a primary T cell response
- Migrate through tissues, track to T cells dependent areas of lymph node and cluster with T cells
- Dendritic cells have unique capabilities to take up antigen by pathways involving phagocytosis, macropinocytosis and clathrin-coated pits
- Cell-surface antigen phenotype distinguishing it from Monocytes/macrophages and B cells
- Cell-surface expression pattern according to their differentiation/maturation/activation
- Main function is priming of T helper cells
- Produces chemokines
Maturation signals
- Exogenous
- Bacteria or their products (LPS, LTA, lipoproteins)
- Viruses or their products (dsRNA, G-RSV)
- Protozoa or their products
- Helminths (SEA, ES 62)
- Endogenous
- Inflammatory mediators (IL-1/TNF-a, hsp, FcR)
- Immune cells (CD40L, CD47, FasL)
Antigen Presentation by Dendritic Cells
- Dendritic cells present antigen to T cells
- Circulating Monocytes differentiate to form immature dendritic cells called Langerhans Cells
- Langerhans cells sample the tissue fluid by endocytosis
- Foreign organisms are internalised
- Within the dendritic cells, antigen is digested to peptides
- Some of the peptides formed bind to the cell’s MHC molecules
- The Langerhans cells leave the epithelium and travel via the afferent lymph. They are now called Veiled Cells
- Veiled cells enter the paracortical region of the lymph node where they present antigen to T cells. They are now called Interdigitating Dendritic Cells
TH1 Cells
- TH1 cells help macrophages digest bacteria (organisms in vesicles)
- TH1 cells secrete a range of cytokines.
- Cytokines secreted include:
- IL-2.
- Gives proliferation of both CD4+ and CD8+ T-cells.
- This stimulation of proliferation of T cells is the main function of the TH1 cell.
- Interferon gamma (IFNγ).
- Activates tissue macrophages
- Is the principal effector mechanism in the defence against intracellular bacteria and parasites.
- E.g. Mycobacteria, Brucella, Rickettsia (bacteria) and Leishmania, Coccidia, Babesia (parasites).
- Activates macrophages and stimulates them to produce enzymes triggering the intracellular killing mechanisms, e.g.
- Superoxide dismutase and myeloperoxidase.
- Produce H2O2 and trigger the "superoxide burst".
- Nitric oxide synthase.
- Produces nitric oxide.
- Superoxide dismutase and myeloperoxidase.
- This is another example of the immune system working through the innate immune response.
- Can act to suppress antibody synthesis.
- IL-2.
TH2 Cells
Common Functions of Th1 and TH2 Cells
- Both TH1 and TH2 cells produce IL-3 and granulocyte-macrophage colony stimulating factor (GM-CSF).
- These act to activate and induce proliferation of neutrophils and also macrophages.
- Neutrophils are the major phagocytic cells in the blood and the principal cells in acute inflammatory lesions.
- Function chiefly in the defence against extracellular bacteria.
- Neutrophils are the major phagocytic cells in the blood and the principal cells in acute inflammatory lesions.
- Therefore, one of the major biological functions of the activation of either TH subset is cytokine-controlled reactive haematopoiesis.
- These act to activate and induce proliferation of neutrophils and also macrophages.
Cytotoxic T-Cells
- Cytotoxic T cells kill virus infected cells (organisms in cytoplasm)
- Viruses are intracellular pathogens that use the host cell machinery for pathogen protein synthesis.
- Viral peptides associate with MHC class I and are expressed on the cell surface.
- CD8+ cytotoxic T-lymphocytes (CTL) recognise the antigen-MHC complex.
- Viral peptides associate with MHC class I and are expressed on the cell surface.
- Cytotoxic T-cells secrete a pattern of cytokines similar to that of TH1 cells.
- I.e. IFNγ but not IL-2.
- The IFNγ shifts the balance of the immune response in favour of TH1 cells.
- There is therefore an increased level of T-cell proliferation.
- The initiation of the immune response via CTL leads to the selective proliferation of CTL - enhances the main mechanism of killing of virally-infected cells.
- There is therefore an increased level of T-cell proliferation.
- The IFNγ shifts the balance of the immune response in favour of TH1 cells.
- I.e. IFNγ but not IL-2.
Killing Mechanism
- The CTl killing mechanism is initiated by direct CTL-target cell contact.
- The cells involved bind by antigen/MHC class I-TcR interaction.
- This results in the CTL's intracellular granules becoming localised to the area of contact.
- These granules contain most of the molecules responsible for cytotoxicity.
- This results in the CTL's intracellular granules becoming localised to the area of contact.
- Direct cell contact stimulates the release of the granule contents into the area of contact between the two cells.
- The granules contain two groups of cytotoxic molecules.
- Perforin.
- Structurally related to the complement component, C9.
- Forms pores in the cell membrane.
- Granzymes.
- Proteolytic enzymes.
- Target cell nucleases.
- Cause programmed cell death.
- Perforin.
T-Cell Activation
- T cells function only after recent activation by antigen.
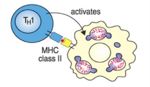
- CD4 binds MHC class II.
- CD4+ T-cells therefore recognise antigen only in association with MHC class II
- CD8 binds MHC class I.
- CD8+ T-cells recognise antigen only in association with MHC class I.
- Activation of T cells requires two distinct signals.
- Signal 1
- The interaction of the TcR with the antigenic peptide/MHC complex on the antigen presenting cell.
- Signal 2
- The interaction of CD28 on the T cells with its ligand, CD80, on the antigen-presenting cell.
- APC expression of CD80 only occurs after:
- The engagement of pattern recognition or Fc receptors.
- Activation with cytokines.
- Interferons, IL-1β or TNFα.
- Therefore signal 2 only occurs after the recognition of danger.
- APC expression of CD80 only occurs after:
- The interaction of CD28 on the T cells with its ligand, CD80, on the antigen-presenting cell.
- Signal 1
Scenarios
- No signal 1
- T cells is not activated as there is no antigen.
- Both signal 1 and signal 2
- T cells is activated into clonal expansion.
- Produces cytokines or becomes cytotoxic.
- This is the response to a dangerous antigen.
- T cells is activated into clonal expansion.
- Signal 1 but no signal 2
- T-cell is triggered into apoptosis and dies.
- This is the basis of "clonal deletion".
- A major mechanism of tolerance.
- Ensures that T-cells do not react with self (non-dangerous) antigens.
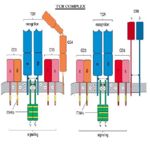
TCR Complex
- The TCR is always associated with CD3
- The TCR and CD3 molecule is referred to as the TCR complex
- TCR is expressed on the surface of T cells in noncovalent association with a complex of transmembrane polypeptides
- CD3 contains 3 distinct polypeptide chains:
- γ, ε, and δ
- Members of the Ig superfamily
- The ε chain associates with both γ and δ
- chaperone role in transporting newly synthesized TCR molecules to the cell surface
- Expressed exclusively on T cells
- CD3 also contains 2 identical chains:
- ζ
- 16 kDa
- Found on T cells, macrophages and NK cells
- Mice also can have an ε (eta) form
Response to Activation
- The response of the T cells to obtaining Signals 1 and 2 is:
- To express the receptor for the cytokine interleukin-2 (IL-2).
- In CD4+ T-cells only, the secretion of IL-2.
- The final trigger for clonal expansion is the engagement of IL-2R with IL-2.
- The IL-2 can come from any activated CD4+ T-cell.
- IL-2 produced by a CD4+ cell may also stimulate clonal expansion of that cell.
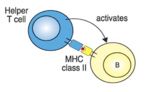
T-Helper Cell Function
- The function of T helper cells is to regulate the immune response.
- The cytokines they secrete exert their influence on other cell populations.
- Most of the different effector cells of the immune system are affected by one or more of the cytokines secreted by TH cells.
- The cytokines they secrete exert their influence on other cell populations.
- TH cells secrete cytokines for only a short period after they have been activated.
- The range of cytokines that TH cells secrete after activation chiefly determines their function.
- Different T-helper cell subpopulations secrete different sets of cytokines.
- Th1 and TH2 cells.
- Different T-helper cell subpopulations secrete different sets of cytokines.