Difference between revisions of "Vaccines"
| (27 intermediate revisions by 4 users not shown) | |||
| Line 28: | Line 28: | ||
'''Disadvantages:''' | '''Disadvantages:''' | ||
| − | *Short duration of action; temporary protection is obtained by the administration of preformed [[Immunoglobulins|antibody]] from another individual of the same or of a different species. The acquired | + | *Short duration of action; temporary protection is obtained by the administration of preformed [[Immunoglobulins - Overview|antibody]] from another individual of the same or of a different species. The acquired antibodies are used in combination with [[Adaptive Immune System - Overview#Antigen Recognition|antigen]], and catabolised by the body, meaning protection is gradually lost over time |
*Injection of antiserum may cause an [[Adverse Drug Reactions|allergic response]] | *Injection of antiserum may cause an [[Adverse Drug Reactions|allergic response]] | ||
| − | *Antiserum contains many | + | *Antiserum contains many antibodies, not just the specific [[Immunoglobulins|antibodies]] needed |
| − | '''Types of | + | '''Types of antibodies administered:''' |
| − | *Maternally-derived | + | *Maternally-derived antibodies in [[Materno-Fetal Immunity - Introduction#Passive transfer via colostrum|colostrum]] when there is a [[Failure of Passive Transfer|failure of passive transfer]] of [[Immunoglobulin G]] |
*Antiserum | *Antiserum | ||
| − | **The | + | **The antibodies are used in combination with [[Adaptive Immune System - Overview#Antigen Recognition|antigen]] (and often an adjuvant) which is injected into a host animal |
| − | **The immune system of that animal synthesises | + | **The immune system of that animal synthesises antibodies |
| − | **Repeated injections at intervals increases total [[Immunoglobulins|antibody]] production | + | **Repeated injections at intervals increases total [[Immunoglobulins - Overview|antibody]] production |
| − | **The immunised animal is bled and the serum collected which contains the newly made | + | **The immunised animal is bled and the serum collected which contains the newly made antibodies. The serum is called '''antiserum'''. |
**The serum can then be injected into a different animal to confer passive immunisation | **The serum can then be injected into a different animal to confer passive immunisation | ||
| − | |||
| − | |||
| − | + | Examples of passive immunisation: | |
{| style="width:60%; height:200px" border="1" align=left | {| style="width:60%; height:200px" border="1" align=left | ||
!INFECTION | !INFECTION | ||
| Line 74: | Line 72: | ||
<br> | <br> | ||
[[Image:Active Immunisation.jpg|thumb|right|200px|Active Immunisation - Copyright nabrown RVC]] | [[Image:Active Immunisation.jpg|thumb|right|200px|Active Immunisation - Copyright nabrown RVC]] | ||
| + | |||
===Active immunisation=== | ===Active immunisation=== | ||
| − | Active immunisation requires the administration of antigen so the patient develops | + | Active immunisation requires the administration of antigen so the patient develops their own antibodies to protect against disease. Suitable antigens include: |
*Living organisms | *Living organisms | ||
*Dead organisms | *Dead organisms | ||
| Line 83: | Line 82: | ||
'''Advantages''' | '''Advantages''' | ||
| − | *Long duration of action; once | + | *Long duration of action; once antibody is produced against the antigen, [[B cell differentiation#Memory cells|memory cells]] are formed which continue circulating in the body |
'''Disadvantages''' | '''Disadvantages''' | ||
*The host's immune system needs to evoke an immune response against the antigen which can take a few days | *The host's immune system needs to evoke an immune response against the antigen which can take a few days | ||
| − | *Can require two or more doses to be effective; the first dose initiates the '''priming''' reaction where | + | *Can require two or more doses to be effective; the first dose initiates the '''priming''' reaction where antibody production ceases after a few weeks, but the second and subsequent doses create memory cells which remain in the circulation for a much longer period of time. |
==Vaccine Antigens== | ==Vaccine Antigens== | ||
| Line 96: | Line 95: | ||
LA vaccines produce a superior response to disease than using killed organisms as the dose of antigen is larger and more sustained, and the response takes place at the site of natural infection, producing a greater local response than with killed organism vaccines. Examples include: | LA vaccines produce a superior response to disease than using killed organisms as the dose of antigen is larger and more sustained, and the response takes place at the site of natural infection, producing a greater local response than with killed organism vaccines. Examples include: | ||
| − | *The current vaccine for | + | *The current vaccine for tuberculosis (called BCG) contains an attenuated form of a mycobacteria |
| − | *Vaccines for | + | *Vaccines for leishmaniasis |
*Vaccines for parainfluenza virus 3 of calves is developed to be temperature-sensitive so that it grows at 34 C in the upper respiratory tract but not at 38 C in the lungs | *Vaccines for parainfluenza virus 3 of calves is developed to be temperature-sensitive so that it grows at 34 C in the upper respiratory tract but not at 38 C in the lungs | ||
| Line 112: | Line 111: | ||
==Adjuvants== | ==Adjuvants== | ||
| − | Adjuvants are used with vaccines containing inactivated organisms which alone would only stimulate a weak immune response. Some adjuvants create a depot of antigen at the injection site allowing a steady flow of antigen into the afferent lymph, while others stimulate the immune system to amplify the adaptive immune response to antigens e.g. pathogen-associated molecular patterns (PAMPs). PAMP-like adjuvants assist naive [[ | + | Adjuvants are used with vaccines containing inactivated organisms which alone would only stimulate a weak immune response. Some adjuvants create a depot of antigen at the injection site allowing a steady flow of antigen into the afferent lymph, while others stimulate the immune system to amplify the adaptive immune response to antigens e.g. pathogen-associated molecular patterns (PAMPs). PAMP-like adjuvants assist naive [[T cells|T cell]] priming. |
| − | Different subtypes of [[ | + | Different subtypes of [[T cell differentiation|T helper cells]] are stimulated by different adjuvants, for example: |
| − | *Aluminium salts generate bias [[T cell differentiation#TH2 Cells|T helper II]] responses for | + | *Aluminium salts generate bias [[T cell differentiation#TH2 Cells|T helper II]] responses for antibody-mediated immunity |
*Killed mycobacteria generate IL-12 producing good '''cell'''-mediated immunity | *Killed mycobacteria generate IL-12 producing good '''cell'''-mediated immunity | ||
| Line 132: | Line 131: | ||
The virus life cycle consists of an extracellular phase, a replicative intracellular phase and another extracellular phase spreading viral particles to other cells to begin the life cycle again | The virus life cycle consists of an extracellular phase, a replicative intracellular phase and another extracellular phase spreading viral particles to other cells to begin the life cycle again | ||
| − | Immunity for the extracellular phase requires neutralising [[Immunoglobulins|'''antibody''']]: | + | Immunity for the extracellular phase requires neutralising [[Immunoglobulins - Overview|'''antibody''']]: |
| − | *[[ | + | *[[B cells]] needed |
*[[T cell differentiation#TH2 Cells|T helper type II cells]] needed (for the [[Major Histocompatability Complexes#MHC II|MHC class II pathway]]) | *[[T cell differentiation#TH2 Cells|T helper type II cells]] needed (for the [[Major Histocompatability Complexes#MHC II|MHC class II pathway]]) | ||
*Live, killed and subunit vaccines can be used | *Live, killed and subunit vaccines can be used | ||
| − | Immunity for the intracellular phase requires [[ | + | Immunity for the intracellular phase requires [[T_cells#Cytotoxic_CD8.2B|CD8+ cytotoxic T lymphocytes (CTL)]] and uses the [[Major Histocompatability Complexes#MHC I|MHC class I pathway]]. |
*Only live vaccine can be used to get into cells (entering via the endogenous pathway) | *Only live vaccine can be used to get into cells (entering via the endogenous pathway) | ||
===Immunity to Bacterial Infection=== | ===Immunity to Bacterial Infection=== | ||
| − | *Extracellular bacterial infection needs | + | *Extracellular bacterial infection needs antibody production for [[Complement#Opsonisation|opsonisation]] and to activate the [[Complement|complement pathways]] |
| − | *[[ | + | *[[B cells]] are needed |
*[[T cell differentiation#TH2 Cells|T helper type II cells]] are needed | *[[T cell differentiation#TH2 Cells|T helper type II cells]] are needed | ||
| Line 152: | Line 151: | ||
[[Image:Colostrum Intake.jpg|right|thumb|200px|Colostrum Intake - Copyright Prof Dirk Werling DrMedVet PhD MRCVS]] | [[Image:Colostrum Intake.jpg|right|thumb|200px|Colostrum Intake - Copyright Prof Dirk Werling DrMedVet PhD MRCVS]] | ||
[[Image:Vaccinating puppies with Parvo.jpg|right|thumb|200px|Response to vaccination against canine parvovirus depending on antibody titre of puppies - Copyright Prof Dirk Werling DrMedVet PhD MRCVS]] | [[Image:Vaccinating puppies with Parvo.jpg|right|thumb|200px|Response to vaccination against canine parvovirus depending on antibody titre of puppies - Copyright Prof Dirk Werling DrMedVet PhD MRCVS]] | ||
| − | *Breeding females can be vaccinated so that immunity is passively | + | *Breeding females can be vaccinated so that immunity is passively transferred to their offspring via the [[Materno-Fetal Immunity - Introduction#Passive transfer via colostrum|colostrum]] - this protects neonates for the first 8-12 weeks of life. |
*Vaccination of young animals should be when the natural passive immunity decreases below the threshold for providing protection. Active immunity should then be stimulated so that the animal has sustained protection. If vaccination is given too early, the natural immunity can interfere with immunisation by binding and neutralising the vaccine antigens. | *Vaccination of young animals should be when the natural passive immunity decreases below the threshold for providing protection. Active immunity should then be stimulated so that the animal has sustained protection. If vaccination is given too early, the natural immunity can interfere with immunisation by binding and neutralising the vaccine antigens. | ||
| Line 177: | Line 176: | ||
'''When to Vaccinate''' | '''When to Vaccinate''' | ||
| − | Puppies are usually first vaccinated between 6 to 8 weeks of age; a second vaccination is given | + | Puppies are usually first vaccinated between 6 to 8 weeks of age; a second vaccination is given 3-4 weeks later. Younger puppies (less than 16 weeks old) may require the third booster 3-4 weeks later, making the vaccination schedule to end between 14 to 16 weeks old. Adult dogs need booster vaccination regularly (depending on the specific vaccination and the recommendations of the vaccine manufacturer). |
===Cat Vaccinations=== | ===Cat Vaccinations=== | ||
| Line 194: | Line 193: | ||
'''When to Vaccinate''' | '''When to Vaccinate''' | ||
| − | Kittens are usually vaccinated around 9 weeks old and a second vaccination is given 3 weeks later. Adult cats need booster vaccination regularly (depending on the specific | + | Kittens are usually vaccinated around 9 weeks old and a second vaccination is given 3 weeks later. Adult cats need booster vaccination regularly (depending on the specific vaccination and the vaccine manufacturers recommendations). |
===Rabbit Vaccinations=== | ===Rabbit Vaccinations=== | ||
| Line 205: | Line 204: | ||
'''When to Vaccinate''' | '''When to Vaccinate''' | ||
| − | Rabbits can be vaccinated against [[Myxomatosis]] from 6 weeks of age and VHD from 2½ to 3 months of age. Booster vaccinations are given every 12 months. In areas at high risk of myxomatosis, it is recommended to give myxomatosis boosters at six-monthly intervals. Some myxomatosis vaccines need to given [[Vaccinations_for_Rabbits#Myxomatosis_Vaccination|partially intradermally]]. | + | Rabbits can be vaccinated against [[Myxomatosis|myxomatosis]] from 6 weeks of age and VHD from 2½ to 3 months of age. Booster vaccinations are given every 12 months. In areas at high risk of myxomatosis, it is recommended to give myxomatosis boosters at six-monthly intervals. Some myxomatosis vaccines need to given [[Vaccinations_for_Rabbits#Myxomatosis_Vaccination|partially intradermally]]. |
==Vaccine Failure== | ==Vaccine Failure== | ||
| − | Failures do occur and should be reported on the VMD [http://www.vmd.gov.uk/ 'yellow form' MLA252A]. Circumstances leading to vaccine failures include: | + | Failures do occur and should be reported on the VMD [http://www.vmd.gov.uk/ 'yellow form' MLA252A] if the events occur in the United Kingdom. Vaccine failures in other European Union (EU) Member States, Norway, Iceland and Liechtenstein should be reported to the relevant competent authority where the event occurred using the [http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000176.jsp&mid=WC0b01ac058002ddcb/ EU reporting forms for veterinarians] which are available in each EU language on the [http://www.ema.europa.eu/ European Medicines Agency] website. Circumstances leading to vaccine failures include: |
*Recipient is already infected with the virus or is immunosuppressed and unable to mount an immune response. | *Recipient is already infected with the virus or is immunosuppressed and unable to mount an immune response. | ||
| − | *Break down of the '''cold-chain''' during transport (incorrect storage of vaccines requiring | + | *Break down of the '''cold-chain''' during transport (incorrect storage of vaccines requiring refrigeration) |
*Improper administration (e.g.myxomatosis vaccine) | *Improper administration (e.g.myxomatosis vaccine) | ||
| − | * | + | *Accidental mixing of inactivated and live vaccines in the same syringe |
*Recipient has maternal antibody to the vaccine | *Recipient has maternal antibody to the vaccine | ||
| Line 223: | Line 222: | ||
*Vaccine is damaged during manufacture | *Vaccine is damaged during manufacture | ||
| − | = | + | {{Learning |
| − | + | |flashcards = [[Vaccination Flashcards|Vaccination Flashcards]] | |
| + | |full text =[http://www.cabi.org/cabdirect/FullTextPDF/2009/20093258316.pdf '''Factors influencing vaccine efficacy - a general review.''' Rashid, A.; Rasheed, K.; Akhtar, M.; Pakistan Agricultural Scientists Forum, Lahore, Pakistan, JAPS, Journal of Animal and Plant Sciences, 2009, 19, 1, pp 22-25, 18 ref.] | ||
| − | + | [http://www.cabi.org/cabdirect/FullTextPDF/2009/20093115229.pdf ''' Establishing vaccine protocols - focus on client communication.''' Datz, C.; The North American Veterinary Conference, Gainesville, USA, Small animal and exotics. Proceedings of the North American Veterinary Conference, Orlando, Florida, USA, 17-21 January, 2009, 2009, pp 608-611] | |
| − | [ | ||
| + | [http://www.cabi.org/cabdirect/FullTextPDF/2009/20093115232.pdf '''Feline lifestyle vaccination protocols.''' Lappin, M. R.; The North American Veterinary Conference, Gainesville, USA, Small animal and exotics. Proceedings of the North American Veterinary Conference, Orlando, Florida, USA, 17-21 January, 2009, 2009, pp 621-624, 18 ref.] | ||
| − | + | [http://www.cabi.org/cabdirect/FullTextPDF/2007/20073166574.pdf '''Immunological basis of vaccination.''' Lunn, D. P.; The North American Veterinary Conference, Gainesville, USA, Large animal. Proceedings of the North American Veterinary Conference, Volume 21, Orlando, Florida, USA, 2007, 2007, pp 135-137] | |
| − | |||
| − | [http://www.cabi.org/cabdirect/FullTextPDF/ | ||
| − | [http://www.cabi.org/cabdirect/FullTextPDF/ | + | [http://www.cabi.org/cabdirect/FullTextPDF/2007/20073166575.pdf '''Equine vaccines: what works, what doesn't?''' Lunn, D. P.; The North American Veterinary Conference, Gainesville, USA, Large animal. Proceedings of the North American Veterinary Conference, Volume 21, Orlando, Florida, USA, 2007, 2007, pp 138-140] |
| − | [ | + | |Vetstream = [https://www.vetstream.com/canis/Content/Freeform/fre00859.asp Vaccination Protocol] |
| + | }} | ||
==Links== | ==Links== | ||
| − | + | :[[:Category:Viral Organisms|Viruses A to Z]] | |
| − | + | :[[:Category:Bacterial Organisms|Bacteria A to Z]] | |
| − | |||
| − | [[:Category:Viral Organisms|Viruses A to Z]] | ||
| − | |||
| − | [[:Category:Bacterial Organisms| | ||
==References== | ==References== | ||
'''Textbooks''' | '''Textbooks''' | ||
| − | |||
*Ivan Roitt: '''Essential Immunology,''' Ninth edition | *Ivan Roitt: '''Essential Immunology,''' Ninth edition | ||
'''Lecture Notes''' | '''Lecture Notes''' | ||
| − | |||
*Dr Brian Catchpole BVetMed PhD MRCVS | *Dr Brian Catchpole BVetMed PhD MRCVS | ||
| − | |||
*Dr Peter H Russell BVSc MSc PhD MRCVS FRCPath | *Dr Peter H Russell BVSc MSc PhD MRCVS FRCPath | ||
| + | <br><br> | ||
| + | {{Jim Bee 2007}} | ||
[[Category:Immunology]] | [[Category:Immunology]] | ||
Latest revision as of 17:02, 4 June 2016
Introduction
Why do we vaccinate animals?
- To protect against infectious diseases
- Where there is no effective treatment once infected e.g. FeLV, FIV
- Where disease is life-threatening e.g. Canine Parvovirus
- To prevent the spread of disease by virus excretion e.g. Rabies, FMDV
The goal is to vaccinate 90% of the population to reduce the amount of endemic virus until no new infections occur. Once the disease risk is low, vaccination can be replaced by an eradication or quarantine programme.
How do vaccines work?
Vaccination induces an immunological memory of the infectious organism. High levels of cytotoxic T cells and neutralising antibody are activated 24 - 48 hours post vaccination as a secondary response (instead of 4-10 days later as a primary response). Neutralising antibody then blocks the attachment of the infectious organism to host cell receptors.
Endogenous vaccines cause antigens to be made as new proteins by the cell, bacterium or virus and involves MHC class I processing live virus, recombinant virus or DNA vaccines.
Exogenous vaccines are when the antigen is processed from the outside by endocytosis without any new proteins being made by the host cell. This involves MHC class II processing inactivated and subunit vaccines.
Route of Administration
- Usually by subcutaneous injection for systemic protection. Some vaccines such as the myxomatosis vaccine NobivacTM Myxo (Intervet UK Ltd) require an intradermal injection as part of the administration procedure.
- For a localised mucosal immune response, intranasal administration is required (IgA) e.g. kennel cough vaccine.
Vaccination Options
Passive immunisation
Advantages
- Immediate protection
Disadvantages:
- Short duration of action; temporary protection is obtained by the administration of preformed antibody from another individual of the same or of a different species. The acquired antibodies are used in combination with antigen, and catabolised by the body, meaning protection is gradually lost over time
- Injection of antiserum may cause an allergic response
- Antiserum contains many antibodies, not just the specific antibodies needed
Types of antibodies administered:
- Maternally-derived antibodies in colostrum when there is a failure of passive transfer of Immunoglobulin G
- Antiserum
- The antibodies are used in combination with antigen (and often an adjuvant) which is injected into a host animal
- The immune system of that animal synthesises antibodies
- Repeated injections at intervals increases total antibody production
- The immunised animal is bled and the serum collected which contains the newly made antibodies. The serum is called antiserum.
- The serum can then be injected into a different animal to confer passive immunisation
Examples of passive immunisation:
| INFECTION | HUMAN SOURCE OF ANTIBODY | EQUINE SOURCE OF ANTIBODY | USE |
|---|---|---|---|
| Tetanus Diptheria | Used | Used | Prophylaxis treatment |
| Botulism | Not used | Used | Treatment |
| Venomous bite | Not used | Used | Treatment |
| Rabies | Used | Not used | Post-exposure to virus |
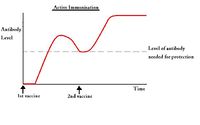
Active immunisation
Active immunisation requires the administration of antigen so the patient develops their own antibodies to protect against disease. Suitable antigens include:
- Living organisms
- Dead organisms
- Toxoids
- Subunit antigens
- DNA
Advantages
- Long duration of action; once antibody is produced against the antigen, memory cells are formed which continue circulating in the body
Disadvantages
- The host's immune system needs to evoke an immune response against the antigen which can take a few days
- Can require two or more doses to be effective; the first dose initiates the priming reaction where antibody production ceases after a few weeks, but the second and subsequent doses create memory cells which remain in the circulation for a much longer period of time.
Vaccine Antigens
Potential antigenic substances include:
Whole Organism
Live Attenuated (LA) vaccines include the organism but in an altered form - virulent organisms cannot be used as vaccines as they have the potential to cause disease. Virulence is reduced by growing the organism in altered conditions (e.g. in cells or eggs), so that it is less able to replicate when introduced into the host, and is therefore less likely to cause disease. Virulence can also be reduced by genetic engineering, or by using naturally occurring avirulent strains.
LA vaccines produce a superior response to disease than using killed organisms as the dose of antigen is larger and more sustained, and the response takes place at the site of natural infection, producing a greater local response than with killed organism vaccines. Examples include:
- The current vaccine for tuberculosis (called BCG) contains an attenuated form of a mycobacteria
- Vaccines for leishmaniasis
- Vaccines for parainfluenza virus 3 of calves is developed to be temperature-sensitive so that it grows at 34 C in the upper respiratory tract but not at 38 C in the lungs
Killed inactivated organism or toxin (toxoid) are useful where virulent and toxic organisms cannot be used as vaccines as they would cause disease. Organisms can be killed using radiation or chemicals so that they still possess the antigens to stimulate an immune response, but the organisms are unable to replicate inside the host. Alternatively, toxins are inactivated to produce a toxoid which will still have the antigens needed to produce an immune response but will not be harmful to the host. Two doses are required to prime the immune system initially, and then induce an immunoligical memory of the disease causing organism.
1:4000 formaldehyde is used in the preparation of killed vaccines; inactivants containing azuridines and beta propiolactone are being developed which do not leave a persistent infectious viral fraction (like formaldehyde).
Subunits (part of the organism)
These can be purified proteins such as a single envelope protein separated from a purified virus by detergent and then centrifuged (traditional method) - genetic engineering can now make single protein vaccines quickly and accurately.
Recombinant or synthetic proteins can also be used as a subunit - the gene for the antigen required is inserted into a virus vector or cloned into bacteria allowing endogenous expression of the antigen. Small antigens, such as peptides, can be produced synthetically where necessary e.g. with Influenza viruses that are constantly mutating, and Canary pox vaccines encoding rabies or FeLV spike proteins (canary pox is safe as it undergoes incomplete replication in mammalian skin cells).
Subunit antigens can also be isolated using the DNA coding for antigenic proteins; circular DNA plasmids are expanded in disabled E.coli strains and then purified - the plasmids expressing the foreign gene can be vaccinated directly into the host.
Adjuvants
Adjuvants are used with vaccines containing inactivated organisms which alone would only stimulate a weak immune response. Some adjuvants create a depot of antigen at the injection site allowing a steady flow of antigen into the afferent lymph, while others stimulate the immune system to amplify the adaptive immune response to antigens e.g. pathogen-associated molecular patterns (PAMPs). PAMP-like adjuvants assist naive T cell priming.
Different subtypes of T helper cells are stimulated by different adjuvants, for example:
- Aluminium salts generate bias T helper II responses for antibody-mediated immunity
- Killed mycobacteria generate IL-12 producing good cell-mediated immunity
Adjuvants decrease the number of injections needed and the amount of antigen that needs to be administered, but they have been associated with vaccine reactions.
Marker Vaccines
Marker vaccines distinguish infected from vaccinated animals in disease control programmes. They contain a deleted protein or gene; vaccinated animals cannot make antibody to the missing protein whereas infected animals can and this helps immunosurveillance for animals infected by an organism in countries that vaccinate against that disease.
Tailoring Vaccines for Specific Diseases
- The life-cycle of infectious organisms needs to be understood to ascertain the best type of immune response for fighting that particular infection
- A vaccine can be created to provide the specific immunity best suited for fighting the associated infection
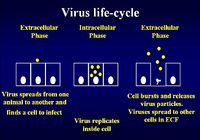
Immunity to Viral Infection
The virus life cycle consists of an extracellular phase, a replicative intracellular phase and another extracellular phase spreading viral particles to other cells to begin the life cycle again
Immunity for the extracellular phase requires neutralising antibody:
- B cells needed
- T helper type II cells needed (for the MHC class II pathway)
- Live, killed and subunit vaccines can be used
Immunity for the intracellular phase requires CD8+ cytotoxic T lymphocytes (CTL) and uses the MHC class I pathway.
- Only live vaccine can be used to get into cells (entering via the endogenous pathway)
Immunity to Bacterial Infection
- Extracellular bacterial infection needs antibody production for opsonisation and to activate the complement pathways
- B cells are needed
- T helper type II cells are needed
Vesicular infections can only be cured by organisms being destroyed inside macrophages
- T helper type I cells are needed
When do we vaccinate?
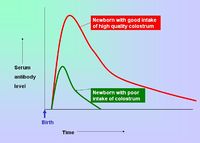
- Breeding females can be vaccinated so that immunity is passively transferred to their offspring via the colostrum - this protects neonates for the first 8-12 weeks of life.
- Vaccination of young animals should be when the natural passive immunity decreases below the threshold for providing protection. Active immunity should then be stimulated so that the animal has sustained protection. If vaccination is given too early, the natural immunity can interfere with immunisation by binding and neutralising the vaccine antigens.
- Two vaccines are usually given to allow for differences between individual animals in the time taken for any natural immunity to decrease.
Dog Vaccinations
Diseases routinely covered by vaccination include:
When to Vaccinate
Puppies are usually first vaccinated between 6 to 8 weeks of age; a second vaccination is given 3-4 weeks later. Younger puppies (less than 16 weeks old) may require the third booster 3-4 weeks later, making the vaccination schedule to end between 14 to 16 weeks old. Adult dogs need booster vaccination regularly (depending on the specific vaccination and the recommendations of the vaccine manufacturer).
Cat Vaccinations
Diseases covered by Vaccination
- Feline Infectious Enteritis (Feline Panleucopenia)
- 'Cat Flu', including Feline Herpesvirus and Feline Calicivirus
- Feline Leukaemia Virus (FeLV)
- Feline chlamydiosis (Chlamydophila felis)
When to Vaccinate Kittens are usually vaccinated around 9 weeks old and a second vaccination is given 3 weeks later. Adult cats need booster vaccination regularly (depending on the specific vaccination and the vaccine manufacturers recommendations).
Rabbit Vaccinations
Diseases covered by Vaccination
When to Vaccinate Rabbits can be vaccinated against myxomatosis from 6 weeks of age and VHD from 2½ to 3 months of age. Booster vaccinations are given every 12 months. In areas at high risk of myxomatosis, it is recommended to give myxomatosis boosters at six-monthly intervals. Some myxomatosis vaccines need to given partially intradermally.
Vaccine Failure
Failures do occur and should be reported on the VMD 'yellow form' MLA252A if the events occur in the United Kingdom. Vaccine failures in other European Union (EU) Member States, Norway, Iceland and Liechtenstein should be reported to the relevant competent authority where the event occurred using the EU reporting forms for veterinarians which are available in each EU language on the European Medicines Agency website. Circumstances leading to vaccine failures include:
- Recipient is already infected with the virus or is immunosuppressed and unable to mount an immune response.
- Break down of the cold-chain during transport (incorrect storage of vaccines requiring refrigeration)
- Improper administration (e.g.myxomatosis vaccine)
- Accidental mixing of inactivated and live vaccines in the same syringe
- Recipient has maternal antibody to the vaccine
- Immunity waning due to missed booster vaccination
- Vaccine is damaged during manufacture
| Vaccines Learning Resources | |
|---|---|
To reach the Vetstream content, please select |
Canis, Felis, Lapis or Equis |
 Test your knowledge using flashcard type questions |
Vaccination Flashcards |
 Full text articles available from CAB Abstract (CABI log in required) |
Factors influencing vaccine efficacy - a general review. Rashid, A.; Rasheed, K.; Akhtar, M.; Pakistan Agricultural Scientists Forum, Lahore, Pakistan, JAPS, Journal of Animal and Plant Sciences, 2009, 19, 1, pp 22-25, 18 ref. |
Links
References
Textbooks
- Ivan Roitt: Essential Immunology, Ninth edition
Lecture Notes
- Dr Brian Catchpole BVetMed PhD MRCVS
- Dr Peter H Russell BVSc MSc PhD MRCVS FRCPath
| Originally funded by the RVC Jim Bee Award 2007 |