Difference between revisions of "Lizard Physical Examination"
| Line 151: | Line 151: | ||
Carry out normal measurement procedures, such as rostrum to [[Cloaca|cloaca]], [[Cloaca|cloaca]] to tail tip and weight. | Carry out normal measurement procedures, such as rostrum to [[Cloaca|cloaca]], [[Cloaca|cloaca]] to tail tip and weight. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[Category:Lizard Examination|C]] | [[Category:Lizard Examination|C]] | ||
Revision as of 11:39, 6 August 2010
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
Consider examining in a quiet area; the use of the oculovagal reflex will allow for a more relaxed clinical examination.
Skin
Examine under a bright light and with a hand lens if necessary. A common problem is retained shed especially on the digits. This may cause avascular necrosis of the digits.
Examine for:
- Ectoparasites
- Trauma due to fighting, mating or thermal burns
- Discoloration (previous scars, injection sites)
- Dysecdysis - retained skin is often dry and brown while normal ecdysis occurring in a piecemeal fashion is flexible and transparent. Retained skin is a focus for infection and acts as a tourniquet on the tail tip and digits leading to ischaemic necrosis
- Examine the scales for loss, scabs, blisters and haemorrhages, ensuring the ventral scales are inspected
- Assess for abnormal masses, swellings or bruised areas
- Assess skin elasticity
Skin folding indicates dehydration, cachexia. Assessment of dehydration in reptiles is analogous to mammals. With 5-8% dehydration there is loss of skin elasticity with a wrinkled appearance. With more severe dehydration (10-15%) the eyes are sunken and the mucous membranes become dry and sticky (consider that the oral cavity of most reptiles is relatively dry compared with mammals).
For more information on normal lizard skin anatomy, see Lizard Integument.
Shedding
Shedding is regular and generally occurs in a piecemeal fashion. It occurs about every 4 to 6 weeks, more often for younger animals during the peak growing seasons and more slowly during winter.
For detailed information on shedding in lizards, see Lizard Shedding.
Head
Rostrum
- Trauma and ulcerations due to repeat escape attempts are very common in water dragons.
Nares
- Check the nares are patent and free of discharge (some iguanids excrete salt from nasal glands via the nares).
Eyes
Ophthalmologic Examination
The eyes are usually considered as a barometer of general health and environmental conditions; therefore a full ophthalmologic examination should be performed in all cases.
- Vision can and should be evaluated by observing certain behaviours such as a menace response.
- Following a general assessment of the eye and associated structures (eyelids, tear film, globe), a more detailed examination should be performed using a form of focal illumination and a magnification system.
- Examination of the ocular fundus to assess the retina (fundoscopy) can be carried out using a direct or indirect ophthalmoscope.
- Mydriasis can be attempted either through general anaesthesia or intracameral injection of neuromuscular blocking agents such as curare or d-tubocurarine. These agents can also be applied topically; however their effectiveness may be affected by the variable corneal penetration of the drugs.
- Other diagnostic tools, such as tonometry (measurement of the intraocular pressure), stains, cytology, bacteriology, histopathology and electron microscopy, in addition to routine diagnostic tools (haemotology, biochemistry, radiology and ultrasound) can also be used to detect an ocular disease or underlying problem in lizards.
For more information on the anatomy of the lizard eye, see Lizard Eye.
Tympanum
- Check that there is no abnormal swelling.
For more information on the anatomy of the lizard ear, see Lizard Ear.
Skull and mandible
- Check that there is no abnormal swelling, bony masses, soft jaw bones (esp. MBD).
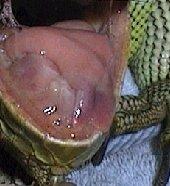
Oral cavity
- Assess the internal extent of rostral abrasions, assess mucous membrane colour and condition (cyanosis? pallor? – normally clear and no petecchiation or oedema).
- Check for excess salivation and stomatitis.
Tongue
- Should be large and fleshy.
Glottis
- Push craniodorsally on the submandibular area to force the glottis up into better view.
- Check for discharges and foreign body.
- Observe during respiration to differentiate between discharges originating from respiratory or GIT.
Pharynx
- Examine using a bright light source.
For more information on the normal anatomy and physiology of the lizard's respiratory system, see Lizard Respiratory System.
For information on the lizard's sense of taste and smell, see Lizard Taste and Smell.
Temporomandibular joint
- Check medial aspects for white deposits (uric acid deposits).
Limbs
- Assess the tone and strength of skeleton and muscle.
- Palpate for abnormal swelling or masses, especially in the joints.
- Decide if swellings are bone (fibrous periosteal reactions or fractures) or soft tissue (includes abscesses).
- Flex and extend joints individually and assess for range of movement and pain.
- Check digits for retained skin and digital necrosis.
- Assess withdrawal reflex by pinching a digit.
Note: Muscle twitching may be associated with MBD.
Coelomic cavity (thorax/abdomen)
- Requires a silent examination area for auscultation. Place stethoscope ventrally between forelimbs or laterally in axilla region for heart and lateral thorax for lungs. A thin, damp cloth between stethoscope and scales aids auscultation. Cardiovascular problems are not uncommon due to calcification of bronchi and major arteries.
- Palpate abdomen for abnormal swellings and masses (e.g. foreign bodies, cystic calculi, constipation, enlarged kidneys). Enlarged kidneys may be palpated ventrally just cranial to the pelvic inlet.
- Smaller lizards (e.g. geckos) can be transilluminated. A powerful light (e.g. endoscope) held against the body of smaller lizards or inserted into the oesophagus or cloaca of larger lizards. Care is necessary if light source produces heat. It is possible to visualise internal structure (e.g. coelomic masses).
Cloaca / tail base
- Check that the cloaca is free from faecal staining, discharge, oedema, erythema, masses or infection. The cloaca can be examined in larger species with a lubricated, gloved finger.
- Enlarged kidneys may be palpated by this method. Endoscopic examination should be considered.
- Examine the tail for swellings, masses, tail tip necrosis and evidence of tail loss and regeneration.
Sexing
Gender identification in juvenile animals may be challenging; however, most lizard species display sexual dimorphism as adults.
- The most reliable method of determining gender is usually by comparing pore sizes. Indeed, adult male lizards tend to have large femoral and precloacal pores on the ventral aspect of the thighs; on the other hand, adult females have smaller, more discrete pores, similar to those seen in juvenile male lizards.
- Sexing probes (cloacal probing) can also be used in iguanas and monitors but aren't as consistently accurate as they are in snakes.
- Injecting saline solution (hydrostatic eversion) into the base of the tail in order to evert the hemipenis is another technique. However, care must be taken to avoid trauma to the hemipenis and prevent pressure necrosis. This method can be useful for species that are difficult to sex such as monitors, Tegus, large skinks, Beaded Lizards and Gila Monsters.
- Temporary eversion of the hemipenes in anaesthetized males can be done by applying gentle pressure at the base of the tail, just caudal to the cloaca.
- In some species, such as iguanas, the hemipenes in mature males will calcify and appear visible on radiographs.
- Endoscopy can be used to visualize gonads.
- Ultrasonic evaluation of the gonads or hemipenes is also possible.
For information on the reproductive anatomy of lizards, see Lizard Reproductive System.
Measurement
Carry out normal measurement procedures, such as rostrum to cloaca, cloaca to tail tip and weight.